Abstract
BACKGROUND: High-frequency percussive ventilation (HFPV) is an alternative mode of mechanical ventilation that has been shown to improve gas exchange in subjects with severe respiratory failure. We hypothesized that HFPV use would improve ventilation and oxygenation in intubated children with acute bronchiolitis.
METHODS: In this single-center prospective cohort study we included mechanically ventilated children in the pediatric ICU with bronchiolitis 1–24 months old who were transitioned to HFPV from conventional invasive mechanical ventilation from November 2018–April 2020. Patients with congenital heart disease, on extracorporeal membrane oxygenation (ECMO), and with HFPV duration < 12 h were excluded. Subject gas exchange metrics and ventilator parameters were compared before and after HFPV initiation.
RESULTS: Forty-one of 192 (21%) patients intubated with bronchiolitis underwent HFPV, and 35 met inclusion criteria. Median age of cohort was 4 months, and 60% were previously healthy. All subjects with available oxygenation saturation index (OSI) measurements pre-HFPV met pediatric ARDS criteria (31/35, 89%). Mean CO2 decreased from 65.4 in the 24 h pre-HFPV to 51 (P < .001) in the 24 h post initiation. SpO2/FIO2 was significantly improved at 24 h post-HFPV (153.3 to 209.7, P = .001), whereas the decrease in mean OSI at 24 h did not meet statistical significance (11.9 to 10.2, P = .15). The mean peak inspiratory pressure (PIP) decreased post-HFPV from 29.7 to 25.0 at 24 h (P < .001). No subjects developed an air leak or hemodynamic instability secondary to HFPV. Two subjects required ECMO, and of these, one subject died.
CONCLUSIONS: HFPV was associated with significant improvement in ventilation and decreased exposure to high PIPs for mechanically ventilated children with bronchiolitis in our cohort and had a potential association with improved oxygenation. Our study shows that HFPV may be an effective alternative mode of ventilation in patients with bronchiolitis who have poor gas exchange on conventional invasive mechanical ventilation.
- high-frequency percussive ventilation
- pediatric ARDS
- mechanical ventilation
- pediatric ICU
- acute bronchiolitis
Introduction
Acute viral bronchiolitis is a common and well-known cause for admission in pediatric ICUs (PICUs) and demands significant health care utilization, accounting for approximately 17% of all hospitalizations for children younger than 2, of whom 5.4% require mechanical ventilation.1 Critically ill children with bronchiolitis are admitted to the PICU when advanced respiratory support is needed to correct hypoxia and/or hypercarbia and can be challenging to manage as they can display rapidly shifting airway dynamics that range from obstructive disease due to small airway plugging to restrictive disease secondary to parenchymal inflammation.2 Furthermore, correcting severe oxygenation and ventilation defects may expose patients to toxic ventilatory settings and ventilator-induced lung injury. Despite its high incidence and health care utilization, bronchiolitis has limited data published regarding ventilatory strategies to treat the respiratory derangements it causes.
High-frequency percussive ventilation (HFPV) is a ventilatory mode that has shown success in treating adult and pediatric subjects with oxygenation and/or ventilation derangements refractory to conventional invasive mechanical ventilation but that has not been rigorously evaluated in the bronchiolitis population.3–7 HFPV uses a pneumatically driven system to create a hybrid of conventional and high-frequency ventilation that was first shown to have efficacy in patients with burn-associated inhalation injury, demonstrating enhanced secretion removal, lung recruitment, and gas exchange among both adult and pediatric subjects.8–10 HFPV's application in bronchiolitis may be similarly advantageous given the similar physiology of high secretion burden and mucous plugging. HFPV is limited, however, by the lack of microprocessor control and return tidal volume measurement that are prominent features of modern ventilators. Similar to high-frequency oscillatory ventilation and high-frequency jet ventilation, HFPV has largely been used in the pediatric population as rescue ventilation when conventional ventilation has failed to produce adequate gas exchange.11,12 No standard for rescue ventilation has been developed for critically ill children though, and no mode of ventilation has shown superiority in outcomes.13 In this study, we sought to add to this literature and to evaluate how HFPV affects gas exchange in intubated subjects with acute bronchiolitis. We hypothesized that HFPV would improve both oxygenation and ventilation in bronchiolitis without significant negative hemodynamic effect or complication.
QUICK LOOK
Current Knowledge
High-frequency percussive ventilation (HFPV) is a mode of ventilation that has been shown to improve gas exchange in adults and children with respiratory failure. Its use in populations outside those with inhalation injuries has been infrequently documented, and there is little evidence to guide providers on its operation.
What This Paper Contributes to Our Knowledge
In this study, HFPV in pediatric subjects with acute bronchiolitis was associated with improved ventilation and variable changes in oxygenation without significant adverse events. HFPV may be an alternative ventilatory mode in patients with bronchiolitis and poor gas exchange on conventional invasive mechanical ventilation.
Methods
Study Design and Subject Selection
This was a prospective, single-center, observational cohort study performed at Primary Children's Hospital, a large academic freestanding children's hospital with a 28-bed PICU, from November 2018–April 2020. At the time of this study, the Primary Children's PICU served as the principal PICU for the state of Utah and was a primary referral site for large areas of 5 surrounding states. Subjects were included in the study if they were admitted to the PICU with a primary diagnosis of viral bronchiolitis, as defined by the American Academy of Pediatrics, and were converted from conventional invasive mechanical ventilation to HFPV.14 Exclusion criteria included age > 24 months or < 28 d, any congenital heart disease, HFPV duration < 12 h, and HFPV initiation during extracorporeal membrane oxygenation (ECMO) utilization. The study was approved by the University of Utah and Primary Children's Hospital Institutional Review Board: IRB number 00104888.
HFPV was implemented using the VDR-4 (Percussionaire, Sandpoint, Idaho). Management of conventional and HFPV modes of ventilation, sedation, and supportive treatments were not protocolized during this study; and all treatment decisions, including transition to HFPV, were made by the clinical providers. The predominant conventional invasive mechanical ventilation strategy at our institution involves use of a pressure-regulated volume control mode with a tidal volume target of 6–8 mL/kg and PEEP titrated for optimal oxygenation and lung recruitment. All pressure measurements gathered utilized the VDR-4 digital multimeter that provides average values of peak inspiratory pressure (PIP), mean airway pressure ( ), and PEEP. Though not protocolized, acceptable ventilator parameters during HFPV management are generally agreed upon among providers and are summarized in Supplemental Figure 1 (see related supplementary materials at http://www.rc.rcjournal.com).
), and PEEP. Though not protocolized, acceptable ventilator parameters during HFPV management are generally agreed upon among providers and are summarized in Supplemental Figure 1 (see related supplementary materials at http://www.rc.rcjournal.com).
Our study period overlapped with the beginning of the SARS-CoV-2 pandemic in the United States. All subjects admitted to the PICU with respiratory symptoms during that time were tested for SARS-CoV-2, and no subjects with bronchiolitis tested positive.
Outcomes
Our primary outcome was the subject's oxygenation saturation index (OSI) at 24 h post-HFPV initiation. Secondary outcome variables included SpO2/FIO2 at 24 h post-HFPV initiation, mean arterial or capillary blood gas CO2 in the 24 h post-HFPV, PICU length of stay, time ventilated, time on HFPV, Pediatric Index of Mortality 3 score, air leak during HFPV, use of neuromuscular blockade infusion > 12 h during HFPV, antibiotic use > 48 h prior to or during HFPV, vasopressor use during HFPV, ECMO following HFPV, and mortality.
Data Collection
Demographic and clinical data were collected and manually entered into a REDCap database. History of chronic lung disease was defined as chronic need for home oxygen or ventilator support. History of reactive airway disease was defined as any parental report of wheezing illnesses requiring use of albuterol or documented diagnosis of reactive airway disease. Pediatric Acute Lung Injury Consensus Conference (PALICC) criteria and severity grading were used to identify and classify pediatric ARDS.15 The physicians caring for each subject at time of HFPV initiation were asked the reason for their decision, recorded as ventilation for refractory hypercarbia, oxygenation for refractory hypoxia, oxygenation and ventilation if both factored into the decision, and secretion management if excessive secretions and not gas exchange prompted the change. The reason for HFPV discontinuation was likewise recorded.
Oxygenation variables, OSI and SpO2/FIO2, and ventilator metrics, PIP,  , and PEEP, were compared from immediately prior to HFPV to their values 24 h post-HFPV initiation. The lowest SpO2 in the 2 h prior to each time point was used to calculate OSI and SpO2/FIO2 and were only calculated when SpO2 was ≤ 97%. The aggregate means of capillary or arterial PCO2 in the 24 h before and after HFPV initiation were compared as a measure of ventilation in place of timed gas collection due to the limitations intrinsic to an observational study.
, and PEEP, were compared from immediately prior to HFPV to their values 24 h post-HFPV initiation. The lowest SpO2 in the 2 h prior to each time point was used to calculate OSI and SpO2/FIO2 and were only calculated when SpO2 was ≤ 97%. The aggregate means of capillary or arterial PCO2 in the 24 h before and after HFPV initiation were compared as a measure of ventilation in place of timed gas collection due to the limitations intrinsic to an observational study.
Statistical Methods
Continuous subject characteristics were summarized as mean (SD) and median (interquartile range). Categorical characteristics were summarized as count (%). Gas exchange and ventilator variables were approximately normally distributed and were compared within subjects before and after HFPV initiation using paired t tests. Normality was assessed by reviewing histograms and results from Shapiro-Wilk tests. To visualize the relationship between CO2 and pH levels and HFPV initiation, we constructed scatterplots in Stata version 17.0 (StataCorp, College Station, Texas) that included all blood gas CO2 and pH levels versus time from HFPV initiation and overlaid a Loess curve with 95% CI shading. We note that since this is a descriptive plot the 95% CIs were not adjusted for correlation among repeat measures (and are thus narrower than true 95% CIs). RStudio with R version 4.0 (R Foundation for Statistical Computing, Vienna, Austria) was used to perform all the analyses.
Results
During the study period, there were 192 patients with 195 associated PICU admissions for bronchiolitis resulting in mechanical ventilation. Forty-one (21%) patients had an HFPV run, and none had a second hospitalization with HFPV use during the study period. Six patients were excluded, one for a history of congenital heart disease and 5 for an HFPV run < 12 h. Of those 5, 2 had improvements in respiratory status and were transitioned back to conventional ventilation, and 3 had worsening respiratory status and were transitioned to ECMO. The remaining 35 subjects represented our study cohort.
The demographic and clinical characteristics of the cohort are shown in Table 1. Thirty-one subjects developed pediatric ARDS prior to HFPV initiation, 26% mild, 35% moderate, and 39% severe. The 4 remaining subjects had pre-HFPV SpO2 > 97% and could not have a pre-HFPV OSI calculated to determine presence of pediatric ARDS, though all subsequently had calculable OSIs and met pediatric ARDS criteria post-HFPV. Four subjects were transitioned off HFPV and subsequently had it reinitiated during the same PICU encounter. One subject had an additional HFPV run following readmission to the PICU during the same hospital encounter. No spontaneous air leaks developed due to HFPV in our cohort, though one subject did develop a pneumothorax during chest tube placement for a pleural effusion. Two subjects (6%) used a vasopressor drip during HFPV due to hypotension in the setting of presumed septic shock. Thirty-three subjects (94%) were placed on a continuous neuromuscular blockade of > 12 h in duration. Two subjects (6%) were cannulated onto ECMO following HFPV initiation, and one subject died secondary to complications from cerebral hemorrhage, representing the single mortality in our cohort.
Demographic and Clinical Characteristics of Cohort
Changes in gas exchange and ventilator parameters following HFPV initiation are shown in Table 2. Two subjects did not have oxygenation variables available for analysis as they were transitioned from HFPV prior to 24 h of therapy. OSI was available for calculation in 25 subjects and SpO2/FIO2 in 27. Change in OSI was not significant, though improved in 60% of subjects (15/25). SpO2/FIO2 showed improvement in 59% of subjects (16/27), increasing from a mean of 153.3 pre-HFPV to 209.7 at 24 h post-HFPV (P = .001). Mean PEEP increased from 9.6 to 10.4 (P = .042) and 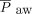 from 15.9 to 18.3 (P < .001).
from 15.9 to 18.3 (P < .001).
Comparison of Gas Exchange and Ventilatory Variables Immediately Prior to High-Frequency Percussive Ventilation Initiation to Those Variables 24 Hours Later
There were 294 blood gases recorded from the 35 cohort subjects in the 24 h before and after HFPV initiation, with a median of 3 samples pre-HFPV and 5 post. Mean PCO2 decreased from 65.4 in the 24 h pre-HFPV to 51.0 post-HFPV (P < .001) (Fig. 1). Scatterplot visualization of all cohort arterial and capillary PCO2 values in the 24 h before and after HFPV is shown in Figure 2. Scatterplot visualization for arterial and capillary pH values is displayed in Supplemental Figure 2 (see related supplementary materials at http://www.rc.rcjournal.com). Mean PIP was found to decrease from 29.7 to 25.0 (P < .001). Ventilator and oxygenation variables for additional collected time points and at time of discontinuation can be found in Supplemental Table 1 (see related supplementary materials at http://www.rc.rcjournal.com).
Box plot of subjects' mean arterial or capillary PCO2 in the 24 h before and after high-frequency percussive ventilation initiation. HFPV = high-frequency percussive ventilation.
Arterial or capillary PCO2 in the 24 h before and after high-frequency percussive ventilation initiation plotted versus time. Loess curve applied with corresponding shaded 95% CIs. HFPV = high-frequency percussive ventilation.
Indications for HFPV are shown in Table 3. Those placed on HFPV for ventilation had an aggregate mean PCO2 of 69 mm Hg in the 24 h pre-HFPV compared to 55 in the oxygenation or secretion management group (P = .005). HFPV was discontinued in 80% (n = 28) of subjects because of improved ventilation, oxygenation, or secretion burden. HFPV was discontinued in 4 subjects (11%) because they failed to improve on HFPV, 2 of whom were cannulated onto ECMO. The remaining 3 subjects were transitioned off HFPV due to various reasons including asynchrony, accidental ventilator disconnection, and iatrogenic pneumothorax during chest tube placement for effusion.
Indications for High-Frequency Percussive Ventilation Initiation and Discontinuation
Discussion
HFPV was associated with significant improvement in ventilation and mixed evidence of improved oxygenation, including no change in our primary outcome of OSI at 24 h, following transition from conventional invasive mechanical ventilation in our study of intubated subjects with acute viral bronchiolitis. Practitioners chose to use HFPV in a variety of clinical situations ranging from rescue ventilation for fulminant pediatric ARDS to management of excessive secretion burden. Improvements in gas exchange were seen without significant complications related to HFPV, with a decrease in potentially injurious PIPs, and in subjects with a wide range of illness severity.
HFPV has been in use for over 30 years, and though its use has not been adopted widely, it has been shown to improve gas exchange in a variety of adult and pediatric populations ranging from those with respiratory failure postcardiac surgery to inhalation injury.3,4,6,7,16–18 Our data suggest HFPV has efficacy in ventilating patients with bronchiolitis, with a mean drop in PCO2 of 14 mm Hg in the 24 h post-HFPV initiation. Studies by Rizkalla,3 Tawfik,6 and Butler7 examined HFPV use in the general pediatric population with respiratory failure and also illustrated improved ventilation, with PCO2 analyzed in their studies at 24, 6, and 2 h post-HFPV, respectively. Our findings may underestimate HFPV's ventilatory efficiency, however, as our comparison does not reflect the weaning of inspiratory pressures that occurs in most subjects post initiation. This is evidenced by the mean Δ pressure (PIP-PEEP) decreasing progressively in our cohort post-HFPV initiation (Supplemental material, Table 1, see related supplemental material at http://www.rcjournal.com).
Though HFPV's effect on ventilation was clear, evidence of improved oxygenation was mixed. SpO2/FIO2 showed a significant improvement with an average increase of 37%; however, the 14% mean decrease in OSI did not reach significance. Our observed significant increases in PEEP and  correlate with the finding of improved SpO2/FIO2. HFPV's contribution to improved SpO2/FIO2 may be fully attributable to this increased distending pressure but may also be related to its removal of airway debris, which has been demonstrated in the inhalation injury population.10,19–21 Improvements in oxygenation from HFPV have been demonstrated in both adults and children in other studies.17,22,23 In the general pediatric population, Rizkalla et al3 found a significant OSI reduction of 39% 24 h post-HFPV initiation; and Tawfik et al6 found a significant increase in SpO2/FIO2 at 6 h post initiation, whereas Butler et al7 did not find any significant change when measuring oxygenation index at 2 h post-HFPV. An important caveat to our gas exchange findings is the parallel observation that initiation of neuromuscular blockade concurrent with HFPV is a standard practice at our institution, with 94% of subjects experiencing an infusion of 12 h or greater. It is possible that changes in gas exchange were attributable to this practice and will need to be carefully evaluated in any future HFPV trial.
correlate with the finding of improved SpO2/FIO2. HFPV's contribution to improved SpO2/FIO2 may be fully attributable to this increased distending pressure but may also be related to its removal of airway debris, which has been demonstrated in the inhalation injury population.10,19–21 Improvements in oxygenation from HFPV have been demonstrated in both adults and children in other studies.17,22,23 In the general pediatric population, Rizkalla et al3 found a significant OSI reduction of 39% 24 h post-HFPV initiation; and Tawfik et al6 found a significant increase in SpO2/FIO2 at 6 h post initiation, whereas Butler et al7 did not find any significant change when measuring oxygenation index at 2 h post-HFPV. An important caveat to our gas exchange findings is the parallel observation that initiation of neuromuscular blockade concurrent with HFPV is a standard practice at our institution, with 94% of subjects experiencing an infusion of 12 h or greater. It is possible that changes in gas exchange were attributable to this practice and will need to be carefully evaluated in any future HFPV trial.
Improved gas exchange during HFPV was achieved in our cohort without necessitating increased peak pressures, producing air leak, or causing hemodynamic instability. Our data show that HFPV can produce high  while simultaneously decreasing high and potentially injurious inspiratory pressures. This is advantageous, as avoidance of high pressures and its associated ventilator-induced lung injury is one of the principal goals of pediatric ARDS management. Additionally, HFPV's ability to achieve higher
while simultaneously decreasing high and potentially injurious inspiratory pressures. This is advantageous, as avoidance of high pressures and its associated ventilator-induced lung injury is one of the principal goals of pediatric ARDS management. Additionally, HFPV's ability to achieve higher  without increasing peak pressures allows providers to better follow an open lung strategy that can be limited by concern for resultant barotrauma and that has been associated with decreased mortality in children.24 This development of improved compliance post-HFPV initiation is likely due to improved recruitment, with mobilization of mucous plugs and re-expansion of collapsed lung segments due to higher
without increasing peak pressures allows providers to better follow an open lung strategy that can be limited by concern for resultant barotrauma and that has been associated with decreased mortality in children.24 This development of improved compliance post-HFPV initiation is likely due to improved recruitment, with mobilization of mucous plugs and re-expansion of collapsed lung segments due to higher  . It should be noted, however, that low tidal volume ventilation cannot be likewise targeted with HFPV due to the lack of tidal volume measurement on the VDR-4 ventilator.15 Though our data suggest a potentially decreased risk of barotrauma from HFPV including no cases of air leak secondary to HFPV, other studies confirm that HFPV can be associated with those findings. Butler et al7 reported an 8% (18/237) incidence of air leak post-HFPV, and Rizkalla3 and Tawfik6 each reported one case in their cohorts (3% and 4%, respectively). Our finding that HFPV does not produce hemodynamic instability in bronchiolitis is supported by similar findings in studies by Oribabor et al25 in adult cardiac subjects and by Rizkalla et al3 and Butler et al7 in children with acute respiratory failure. The pediatric study by Tawfik et al,6 however, did find one case of clinically important hypotension related to HFPV. These results suggest that those placed on HFPV require judicious management of high pressures to avoid resultant complications. Our operating guide shown in Supplemental Figure 1 (see the supplementary materials at http://www.rcjournal.com) was primarily designed for such safety and standardization and may be a useful tool in centers considering offering HFPV to their patients. Mortality was low in our cohort with only a single death and is consistent with the generally low mortality in the bronchiolitis population, reported at 0.06% by Fujiogi et al.1
. It should be noted, however, that low tidal volume ventilation cannot be likewise targeted with HFPV due to the lack of tidal volume measurement on the VDR-4 ventilator.15 Though our data suggest a potentially decreased risk of barotrauma from HFPV including no cases of air leak secondary to HFPV, other studies confirm that HFPV can be associated with those findings. Butler et al7 reported an 8% (18/237) incidence of air leak post-HFPV, and Rizkalla3 and Tawfik6 each reported one case in their cohorts (3% and 4%, respectively). Our finding that HFPV does not produce hemodynamic instability in bronchiolitis is supported by similar findings in studies by Oribabor et al25 in adult cardiac subjects and by Rizkalla et al3 and Butler et al7 in children with acute respiratory failure. The pediatric study by Tawfik et al,6 however, did find one case of clinically important hypotension related to HFPV. These results suggest that those placed on HFPV require judicious management of high pressures to avoid resultant complications. Our operating guide shown in Supplemental Figure 1 (see the supplementary materials at http://www.rcjournal.com) was primarily designed for such safety and standardization and may be a useful tool in centers considering offering HFPV to their patients. Mortality was low in our cohort with only a single death and is consistent with the generally low mortality in the bronchiolitis population, reported at 0.06% by Fujiogi et al.1
Our study did not specify presence of pediatric ARDS as an inclusion criterion, but all subjects in the cohort on whom OSI could be calculated (89%) met that designation pre-HFPV. Despite its use in pediatric ARDS subjects, providers' primary reason for HFPV initiation was ventilation, representing 60–74% of the cohort, as opposed to 11–25% for oxygenation failure and 14% for secretion management. Tawfik et al6 and Butler et al7 also found that ventilation was a more common reason than oxygenation for HFPV initiation, though secretion management was the most common reason given in both studies. In contrast, adult studies show that HFPV's use in those populations is primarily for hypoxemia.18,25–27 This difference is likely reflective of the challenging physiology of bronchiolitis and pediatric respiratory failure, with changing compliance and dangerous hypoxemia often developing rapidly in the setting of mucous plugging of the small and large airways. Practitioners may be targeting hypercarbia and secretions with HFPV to prevent potential hypoxemic crises, and our data would support the vulnerability of this population to those events: 12% (n = 5) of those initiated on HFPV progressed to ECMO, 2 in our cohort and 3 who were on HFPV for < 12 h, including one subject who suffered a pulseless cardiac arrest secondary to mucous plugging.
This study has a number of limitations. As a single-center study, our results do not reflect the experiences of other institutions and may not be easily reproducible given the lack of standardized HFPV management guidelines. The single-center design and resultant lack of equipoise in ventilatory strategy introduce selection bias and do not allow for comparison to control subjects not placed onto HFPV to detect whether improved outcomes can be truly attributed to HFPV. As an observational study, another limitation was the lack of optimally timed and available data to fully detect changes in gas exchange, evidenced by multiple subjects without values to appropriately calculate OSI and mixed capillary and arterial blood gas sampling, which introduces bias into our measurement of HFPV's ventilatory efficacy. The observational design and lack of a comparison group also did not allow us to control for potentially major confounders, such as the presence of continuous neuromuscular blockade, which could have significantly affected our findings. Additionally, because HFPV for bronchiolitis is a relatively uncommon occurrence, our study's results need to be interpreted with caution given the small sample size that may be underpowered to detect rare but important complications of HFPV. Larger multi-center studies will allow for greater standardization in HFPV management as well as further comparison of its efficacy to conventional invasive mechanical ventilation.
Our study strengthens the current literature by evaluating HFPV's use in the most common cause of pediatric respiratory failure, doing so in a prospective fashion that allowed for careful adjudication of data. To our knowledge, this is the only report to date specifically describing HFPV use in detail among those intubated with bronchiolitis. HFPV has become an important tool for subjects in respiratory failure at our institution when other ventilatory methodologies have been unsuccessful in reversing hypercarbia and/or hypoxia and may offer an alternative to continuing escalation of potentially injurious conventional invasive mechanical ventilation settings or initiating the invasive option of ECMO. Our study confirms the efficacy of HFPV in acute bronchiolitis, and our experience has allowed us to create a model of HFPV use that other centers can utilize for this young and vulnerable population.
Conclusions
HFPV initiation was associated with improved ventilation and potentially with improved oxygenation without significant adverse events in our cohort of intubated subjects with acute bronchiolitis. This evidence suggests that HFPV may be an effective rescue therapy for refractory hypercarbia in bronchiolitis and may be lung protective given the lower peak pressures needed to produce improved gas exchange. However, HFPV's utility in addressing refractory hypoxemia is unclear based on these results. Our study improves knowledge of this ventilatory mode's uses and provides a base for future broad research efforts. It will now be important to design rigorous multi-center investigations of HFPV in pediatrics and develop best practices for its use.
Acknowledgments
We would like to thank and acknowledge Allison Neeley for her help in data acquisition for this project; Dave Clegg, Troy Lynch, and Dr Anna Hubbard for sharing their expertise; and the many nurse practitioners, nurses, and respiratory therapists who care for these critically ill children.
Footnotes
- Correspondence: Benjamin White MD, Department of Pediatrics, Penn State Health Children's Hospital, 500 University Drive, Hershey, PA 17033. E-mail: benjaminrobertwhite{at}gmail.com
Supplementary material related to this paper is available at http://rc.rcjournal.com.
The authors have disclosed no conflicts of interest.
A version of this paper was presented by Dr White at the Pediatric Critical Care Summit of the Americas/26th Pediatric Critical Care Colloquium USA, held in Houston, Texas, November 7–10, 2019.
This study was supported in part by the University of Utah Population Health Research Foundation, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant 5UL1TR001067-05 (formerly 8UL1TR000105 and UL1RR025764).
This study was performed at Primary Children's Hospital, Salt Lake City, Utah.
See the Related Editorial on Page 893
- Copyright © 2022 by Daedalus Enterprises









