Abstract
BACKGROUND: Mechanical insufflation-exsufflation (MI-E) devices are used to improve airway clearance in individuals with acute respiratory failure. Some MI-E devices measure cough peak flow (CPF) during MI-E to optimize pressure adjustments. The aim was to compare CPF and effective cough volume (ECV: volume expired/coughed > 3 L/s) measurements between 4 MI-E devices under simulated conditions of stable versus collapsed airway.
METHODS: Four MI-E devices were tested on the bench. Each device was connected via a standard circuit to a collapsible tube placed in an airtight chamber that was attached to a lung model with adjustable compliance and resistance. Pressure was measured upstream and downstream the collapsing tube; air flow was measured between the chamber and the lung model. Each device was tested in 2 conditions: collapse condition (0 cm H2O) and no-collapse condition (−70 cm H2O). For each condition, 6 combinations of inspiratory/expiratory pressures were applied. CPF was measured at the “mouth level” by the device built-in flow meter and at the “tracheal level” by a dedicated pneumotachograph. Comparisons were performed with non-parametric tests.
RESULTS: CPF values measured at the tracheal level and ECV values differed between devices for each inspiratory/expiratory pressure in the collapse and no-collapse conditions (P < .001). CPF values were significantly lower at the tracheal level in the collapse as compared with the no-collapse condition (P < .001 for each device), whereas they were higher at the mouth level (P < .05) for 3 of the 4 devices.
CONCLUSIONS: CPF values differed significantly across MI-E devices, highlighting limitation(s) of using only CPF values to determine cough effectiveness. In simulated of airway collapse, CPF increased at the mouth, whereas it decreased at the tracheal level.
Introduction
Impaired cough ability reduces the ability to clear airway secretions. Mechanical insufflation-exsufflation (MI-E) applied through an endotracheal tube, a tracheostomy tube or a face mask, improves airway clearance in individuals with impaired cough.1,2 MI-E can assist secretion clearance in people with respiratory muscle weakness caused by a wide variety of neuromuscular diseases.1,3 Cough effectiveness is commonly evaluated by measuring expiratory cough peak flow (CPF). CPF is defined as the expiratory peak flow of air generated by the respiratory system during the expulsive phase of a cough maneuver. Among all available procedures to improve cough performance, MI-E produces the greatest increase in CPF.4 The most commonly used MI-E devices monitor CPF via a built-in sensor. However, the human upper airway could be considered as a collapsible tube (pharynx) with upstream and downstream rigid segments, as described by the classic Starling resistor model.5 Thus, when intraluminal pharynx pressure drops below that of the surrounding tissue pressure, collapse occurs, which results in flow limitation.5 Accordingly, upper-airway collapsibility is commonly measured by applying noninvasive negative expiratory pressure of −5 cm H2O or −10 cm H2O during spontaneous expiration during wakefulness.6
When negative expiratory pressure application induces collapse, this is seen as a peak flow spike, resulting mainly from dynamic airway compression, followed by a drop in flow of variable magnitude that is caused by an increase in the resistance of the oropharyngeal structures.6 Therefore, we hypothesized that CPF measurement does not provide an indication of collapsibility, whereas the volume coughed at an efficient flow (> 180 L/min), designated effective cough volume (ECV) herein, would decrease in the event of upper-airway collapse.
Accordingly, the aim of this study was to compare CPF and ECV measurements between 4 MI-E devices under simulated conditions of stable versus collapsed airway. We placed a pneumotachograph at the tracheal level to measure changes in CPF and ECV induced by changes in upper-airway resistance during exsufflation. We also evaluated the CPF values at the mouth level provided by the MI-E devices, which should also be dependent to changes in upper-airway volume. We hypothesized that CPF and ECV values would vary widely between the devices.
QUICK LOOK
Current Knowledge
Cough effectiveness is usually assessed by measuring expiratory cough peak flow (CPF): the peak flow of air generated by the respiratory system during a cough maneuver). Mechanical insufflation-exsufflation (MI-E) is increasingly used to provide cough assistance, and several MI-E devices measure CPF; however, some data suggest that CPF values may be impacted by undetected upper-airway collapse. This could misguide assessment of cough effectiveness when titrating the level of negative pressure during MI-E.
What This Paper Contributes to Our Knowledge
We found large differences in CPF values between MI-E devices. This confirms the limits of only using CPF to assess cough effectiveness: When collapse occurs, CPF paradoxically increases, which falsely suggests improved cough effectiveness.
Methods
Equipment
Four MI-E devices that are available in France, approved for clinical practice, and that provide CPF measurements via a built-in sensor were used in the present study: CoughAssist E70 (Philips Respironics, Murrysville, Pennsylvania), Pegaso (Dima Italia, Bologna, Italy), Comfort Cough II (WILAmed, Kammerstein, Germany), and EOVE-70 (Air Liquide Medical Systems, Antony, France).
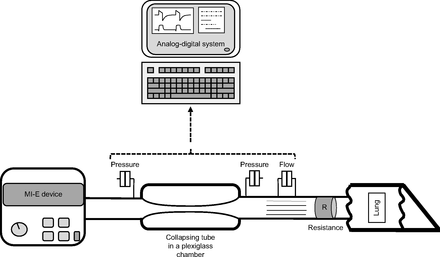
Each MI-E device was connected, via a standard circuit, to the following bench model: A collapsible tube (see below for characteristics) that simulated the upper airway was attached in series to a lung model with adjustable compliance and resistance (Michigan Dual Adult Test Lung TTL 2600i, Michigan Instruments, Grand Rapids, Michigan) (Fig. 1). Because individuals with neuromuscular disorders have both low lung compliance and low elastic chest wall properties,7 the compliance of the lung model was set at only 30 mL/cm H2O. Since these individuals generally have normal airway resistance when the upper airway does not collapse,7 we used the lowest available resistance proposed by Michigan Instruments (5 cm H2O/L/s) (PneuFlo airway resistor Rp5, Michigan Instruments), which we inserted between the chamber and the lung model.
Schematic representation of the setup used to assess the cough-assist devices. Flow was measured between the lung and the collapsible tube to avoid flow induced by the compliance of the tubing. Airway pressure was measured both upstream and downstream of the collapsing tube.
In order to measure expiratory flow from the lungs and not flow generated from the rapid expulsion of the air contained in the upper airway when collapse occured,8 flow signal was measured between the collapsing tube and the PneuFlo airway resistor using a Fleisch pneumotachograph (Fleisch, Lausanne, Switzerland) associated with a pressure differential transducer (Validyne DP 45 +/− 3.5 cm H2O; Validyne, Northridge, California). Airway pressure was measured both upstream and downstream of the collapsing tube using the pressure differential transducers. Flow and pressure signals were sampled at 200 Hz and recorded using an analog-digital system (MP100, Biopac Systems, Goleta, California) and its software (version 3.8).
Characteristics of the Collapsible Tube
The collapsible latex tube has been previously described.9 Briefly it had a diameter of 18 mm, a length of 15 cm, and a thickness of 1 mm (Michelin, Clermont-Ferrand, France), and it was mounted between two 22M-22M straight connectors (Intersurgical, Workingham, England) and surrounded by a plexiglass airtight chamber in which the pressure could be adjusted.
Study Procedures
Experimental Protocol
The experiments were performed in room air at ambient temperature. The flow and pressure transducers were calibrated under these conditions before the measurements. The pneumotachograph was calibrated (volume calibration) using a 3-L syringe. The collapsible tube was inserted in the airtight chamber. For each device, we tested 2 conditions with different levels of pressure around the collapsible tube: a no-collapse condition at −70 cm H2O to maintain a positive transmural pressure within the collapsing tube and a collapse condition at 0 cm H2O to reproduce a situation in which collapse was not induced by positive pressure but was only induced during expiration when a negative pressure was applied to the airway by the MI-E device.
For each pressure chamber condition, we applied pairs of inspiratory/expiratory pressures set at +20/−20 cm H2O, +30/−30 cm H2O, +40/−40 cm H2O, +40/−50 cm H2O, +40/−60 cm H2O, and +40/−70 cm H2O. In each condition, the pressure and flow signals were continuously recorded at 200 Hz. The MI-E was cycled automatically. The insufflation and exsufflation durations were 2 s each, with an intercycle pause set to 1 s. After a stabilization period, at least 10 cycles were analyzed per condition.
Data Analysis
Primary end points were CPF and ECV. The last 10 cycles of each recording were used for the analysis. The analysis was performed with an in-house application developed in MATLAB R2021a that automatically analyzed the files generated by the data logger breath by breath. ECV was derived from the flow-time curve by integration.
An experimenter verified the collapse visually, and each trial was considered effective the first time that an increasing pressure drop across the collapsing tube was not associated with an increase in expiratory flow. The time from the beginning of expiration and this criterion (δ-time collapse: DTcol) was also automatically analyzed.
Statistical Analysis
Data were described as mean ± SD. Because of non-normality of residuals with analysis of variance, we performed a nonparametric factorial analysis using the aligned rank transform technique. Moreover, because we expected an interaction between device effect, collapse effect, and inspiratory/expiratory pressures, a Kruskal-Wallis test was performed for each inspiratory/expiratory pressure combination in the collapse condition (0 cm H2O or −70 cm H2O chamber pressure, respectively) to compare the devices and the conditions (collapse and no-collapse), followed by pairwise Mann-Whitney comparisons with a Holm correction.
CPF measurement was performed with independent/different built-in flow meters for each device; therefore, CPF was expressed as a percentage of the value obtained with inspiratory/expiratory pressures of +20/−20 cm H2O in the no-collapse condition (−70 cm H2O chamber pressure). Data were compared between conditions (collapse/no-collapse) with a Wilcoxon test for paired data for each device.
Statistical significance was set at .05 (2-tailed test). Statistical analysis was performed using R statistical software version 2.2.0 and ART package.
Results
Collapse did not occur in the no-collapse condition (−70 cm H2O chamber pressure); however, it occurred systematically in the collapse condition when the pressure around the collapsible tube was maintained at 0 cm H2O. DTcol values are presented in Table 1 for this condition, for each device, and each expiratory pressure adjustment.
Values of the Time From the Beginning of Expiration to the First Instant That an Increase in Pressure Drop Across the Collapsing Tube in the Collapse Condition
Comparison of Cough Peak Flow at the “Tracheal” Level Between Devices
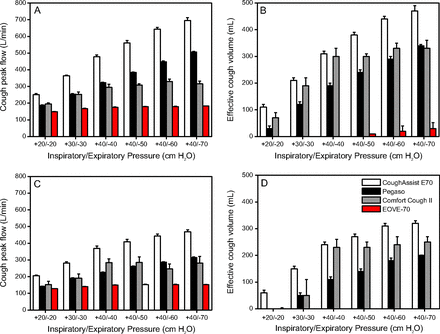
Figure 2A shows CPF values for each device at each inspiratory/expiratory pressure level in the collapse and no-collapse conditions. CPF values for all devices differed significantly between inspiratory/expiratory pressure levels (P < .001).
Mean cough peak flow (A and C) and effective cough volume (B and D), defined as the volume exhaled > 180 L/min,22 measured for each mechanical insufflation-exsufflation device at each inspiratory/expiratory pressure for both the collapse (0 cm H2O) (C and D) and no collapse (−70 cm H2O) (A and B) conditions. Error bars denote SD.
CPF values were lower in the collapse than the no-collapse condition for all devices (P always < .02) except Comfort Cough II at +40/−40 cm H2O and +40/−50 cm H2O (P = .27 and P = .36, respectively).
In the no-collapse condition, CPF values were significantly higher for the CoughAssist E70 than the other devices (P < .001), followed by Pegaso versus Comfort Cough II (P < .001, except for +20/−20 cm H2O P = .004 and for +30/−30 cm H2O P = .82) and Comfort Cough II versus EOVE-70 (P < .001).
In the collapse condition, CPF values were significantly higher for CoughAssist E70 than the other devices (P always < .001). CPF values did not differ significantly between Pegaso and Comfort Cough II at any of the 3 inspiratory/expiratory pressures. CPF values were lowest for EOVE-70 (P < .001, except for +20/−20 cm H2O P = .006).
Effective Cough Volume
Figure 2B shows ECV values for each device at each level of inspiratory/expiratory pressure in the collapse and no-collapse conditions. The ECV values for all devices differed significantly between inspiratory/expiratory pressure levels in the collapse and no-collapse conditions (P < .001).
ECV was significantly lower in the collapse than the no-collapse condition for each device (P always < .001 except for EOVE-70 +40/−60, P = .01; and EOVE-70 +40/−50 cm H2O, P = .34).
ECV values were significantly higher for CoughAssist E70 than for the other devices in the collapse and no-collapse conditions (P < .001) except Comfort Cough II in the no-collapse condition at +30/−30 cm H2O (P = .11) and +40/−40 cm H2O (P > .99). ECV values were higher for Comfort Cough II than Pegaso (P < .001) except in the no-collapse condition at +40/−70 cm H2O (P = .80) and in the collapse condition at +30/−30 cm H2O (P = .22) and EOVE-70 (P < .001) at all inspiratory/expiratory pressure levels.
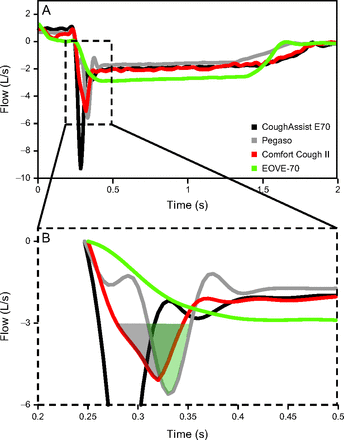
CPF values did not differ significantly between Pegaso and Comfort Cough II, whereas ECV values were significantly higher with Comfort Cough II. Therefore, we performed visual analysis of the flow curves and found that, although the CPF value from Comfort Cough II was greater than that of Pegaso, its duration was almost systematically shorter: This is demonstrated in Figure 3 at +40 cm H2O/−50 cm H2O in the no-collapse condition.
A: Flow-time waveform during exsufflation for the 4 mechanical insufflation-exsufflation devices, with inspiratory/expiratory pressure set at +40/−50 cm H2O, in the no-collapse condition (−70 cm H2O). B: Highlighted area enlarged.
CPF Measured by the Device
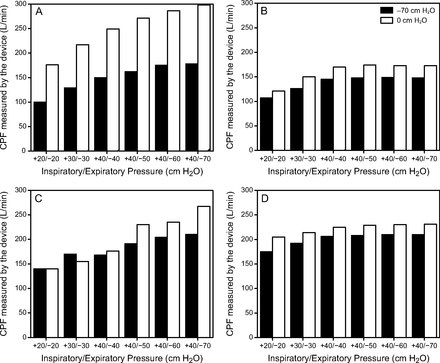
Contrary to the CPF measured between the collapsing tube and the PneuFlo airway resistor, CPF values measured by the devices were generally higher in the collapse condition (0 cm H2O) than the no collapse condition (−70 cm H2O). CPF values were significantly higher when the tube collapsed for each device (P < .05) except Pegaso (P = .18) (Fig. 4).
Cough peak flow (CPF) measurements obtained for the 4 mechanical insufflation-exsufflation devices for all the inspiratory/expiratory pressures in the collapse (0 cm H2O) and no-collapse (−70 cm H2O) conditions. A: CoughAssist E70, B: Comfort Cough II, C: Pegaso, and D: EOVE-70.
Discussion
CPF has been widely used to evaluate cough effectiveness with and without cough assistance techniques.4 New MI-E devices measure CPF4; however, the use of CPF values to measure cough effectiveness is limited by a lack of agreement between the measurements from different devices.10-13 This between-device discrepancy mainly results from differences in frequency responses, that is, how quickly the measurement device can respond to an instantaneous full-scale flow change.14
For this reason, we compared 4 different MI-E devices with the same pneumotachograph that was connected to a high-frequency response pressure transducer. We also performed paired comparisons of CPF values measured at the mouth by the devices in collapsing and non-collapsing conditions, independently from the MI-E device used.
Lachal et al9 were the first to demonstrate with a bench model that, when the mechanical properties of the subglottic respiratory system and the insufflation/exsufflation settings remain the same, upper-airway collapse during exsufflation increases CPF at the mouth level. We confirmed this result using CPF measurements taken directly by the MI-E devices (Fig. 4). In contrast, CPF measured at the tracheal level (ie, between the collapsible tube and the lung model) decreased when collapse occurred. This difference in CPF pattern upstream and downstream of the upper airway during collapse can be attributed to a decrease in upper-airway volume during exsufflation that increases CPF at the “mouth” level and decreases CPF at the tracheal level by increasing airway resistance. These results highlight the limits of only using CPF to determine cough effectiveness when a negative pressure is applied through a face mask during expiration to assist the expiratory muscles. Our bench study confirmed that CPF values measured by MI-E devices are overestimated relative to peak flow at the lung levels in the situation of upper-airway collapse.
MI-E device performance is affected by the mechanical properties of the respiratory system.15 However, mechanical properties and, especially, airway resistance can change depending on the potential for upper-airway collapse, which may also depend on the MI-E device settings. Therefore, the aim of this study was to compare the devices in conditions of collapse and no-collapse. Except for the 2 devices that generated intermediate results and whose performances were similar, the devices that generated higher CPF values also generated higher ECV values. Visual comparison of the flow behavior of the devices that produced intermediate results with exsufflation applied revealed that one device generated a smaller peak amplitude but that was longer in duration, which explained the apparently paradoxical results. Similarly, visual analysis of the flow-volume curve also explained this apparent contradiction (results not shown). We previously found an increase in CPF values with a paradoxical decrease in ECV values in several individuals with neuromuscular disorders when negative expiratory pressure was increased.16 From a mathematical point of view, CPF is the maximum value of a variable (flow), whereas ECV is the integral of this variable during the time that it is above a given threshold. Therefore, CPF and ECV are not necessarily correlated. Moreover, this apparent paradoxical behavior between CPF and ECV was associated with an expiratory flow curve profile that suggested upper-airway collapse, that is, an abnormal, abrupt fall in expiratory flow after the flow peak and thus a flattening of the flow-volume curve induced by an increase in the negative exsufflation pressure.16
As expected, device performance, in terms of CPF and ECV at the tracheal level, was systematically affected by the upper-airway collapse simulation. We demonstrated in an in vivo study that, when collapse occurs, CPF measured at the mouth level increases while ECV, defined as the volume coughed > 180 L/min, decreases.16 In the present bench study, we found that CPF measured at the tracheal level decreased. Therefore, one can consider that the previously observed faster CPF with MI-E delivered via a face mask rather than an endotracheal tube16 could be the result of (1) a change in airway resistance and (2) the different time points at which MI-E was applied before and after extubation; that is, sedation levels and cooperation may have differed, and secretion volume and lung resistance may have reduced over the study period. We can add that increase in CPF after extubation could also result from upper-airway collapse; this could be identified by measuring ECV.
Limitations
This study has several limitations. First, maximal insufflation pressure was limited to 40 cm H2O. However, we followed the method used by Mellies and Goebel17 who titrated inspiratory pressure until no further volume increment could be achieved. Using this method, they demonstrated that submaximal insufflation (< 40 cm H2O) generates the highest individual CPF, even in individuals with severely reduced respiratory system compliance.
Second, it is challenging to represent a real-life individual in a bench study; and therefore, the CPF and ECV values obtained in our study should be interpreted with caution. However, the values were consistent with those of our previous clinical study.16 Although we consider that we simulated clinical tracheostomy when preventing tube collapse by using −70 cm H2O of pressure around the collapsible tube to prevent collapse, we were not able to simulate an individual’s effort during exsufflation. Nevertheless, our objective was only (1) to demonstrate that the increase in CPF downstream of the collapsed section was paradoxically accompanied by a decrease in CPF upstream of the collapsed section and (2) to compare the MI-E devices in identical conditions, which is not possible in vivo.
Conclusions
This study demonstrates important differences between MI-E devices and underlined the limits of only evaluating CPF to assess cough effectiveness. CPF measurement does not provide an indication of collapsibility, in contrast with flow-volume curve analysis and ECV, as previously suggested.16 Moreover, although CPF values decreased at the tracheal level in the collapse condition, they were paradoxically increased at the mouth level. This could lead clinicians to increase expiratory pressure during MI-E titration, which would be deleterious. We also suggest that the manufacturers’ claim that “the displayed values of CPF may be used to titrate expiratory pressure levels” is misleading. Future MI-E devices should include a presentation of flow-volume curves or ECV values for the detection of upper-airway collapse during MI-E titration.
Footnotes
- Correspondence: Nicolas Terzi MD PhD, Service de Médecine intensive Réanimation, Centre Hospitalier Universitaire Grenoble–Alpes, CS10217, Grenoble Cedex 09, France. E-mail: nicolas.terzi{at}chu-rennes.fr
See the Related Editorial on Page 553
The authors have disclosed no conflicts of interest.
Drs Lofaso and Louis contributed equally to this manuscript.
- Copyright © 2023 by Daedalus Enterprises