Abstract
BACKGROUND: Patients receiving mechanical ventilation commonly experience sleep fragmentation. The present meta-analysis compared the effects of pressure controlled ventilation (PCV) and pressure support ventilation (PSV) on sleep quality.
METHODS: We conducted a search of the PubMed, Embase, and Cochrane Library databases for studies published before November 2023. In this meta-analysis, individual effect sizes were standardized, and the pooled effect size was determined by using random-effects models. The primary outcome was sleep efficiency. The secondary outcomes were wakefulness, percentages of REM (rapid eye movement) sleep and stages 3 and 4 non–REM sleep, the fragmentation index, and the incidence of apneic events.
RESULTS: This meta-analysis examined 4 trials that involved 67 subjects. Sleep efficiency was significantly higher in the PCV group than in the PSV group (mean difference 15.57%, 95% CI 8.54%–22.59%). Wakefulness was significantly lower in the PCV group than in the PSV group (mean difference −18.67%, 95% CI −30.29% to −7.04%). The percentage of REM sleep was significantly higher in the PCV group than in the PSV group (mean difference 2.32%, 95% CI 0.20%–4.45%). Among the subjects with a tendency to develop sleep apnea, the fragmentation index was significantly lower in those receiving PCV than PSV (mean difference −40.00%, 95% CI −51.12% to −28.88%). The incidence of apneic events was significantly lower in the PCV group than in the PSV group (risk ratio 0.06, 95% CI 0.01–0.45).
CONCLUSIONS: Compared with PSV, PCV may improve sleep quality in patients receiving nocturnal mechanical ventilation.
- mechanical ventilation
- pressure controlled ventilation
- assist-control ventilation
- pressure support ventilation
- sleep quality
Introduction
Patients receiving mechanical ventilation often experience disrupted sleep, characterized by sleep fragmentation.1,2 This condition is influenced by several factors, including a busy clinical environment, frequent patient disturbance by medical staff, repositioning the patient, and suctioning. In addition, patients in ICUs are frequently exposed to bright light and noises from medical equipment, which further compromise their sleep quality.2 Therefore, caregivers should minimize disruptive stimuli during nocturnal mechanical ventilation.
Pressure support ventilation (PSV) is commonly used to wean patients off invasive ventilation, and it can be used during both day and night. However, Parthasarathy et al3 reported that PSV was associated with increased sleep fragmentation. Patients receiving PSV may experience apneic episodes due to ineffective triggering caused by excessive pressure support, which leads to respiratory alkalosis that may reduce respiratory drive and further exacerbates apnea. When apnea occurs, audible alarms are triggered, which arouse patients from sleep. In contrast, pressure controlled ventilation (PCV) is preferred over PSV for nocturnal mechanical ventilation for two reasons. First, PCV maintains a backup rate and delivers continuous inspiratory flow, reducing the likelihood of sleep fragmentation. Second, PCV allows the respiratory muscles to rest, which might improve the endurance of the inspiratory muscles during the weaning process. A study4 and a meta-analysis5 have shown that sleep-promoting interventions can improve both sleep quality and quantity; however, patient baseline characteristics and ventilators with varying support levels were not analyzed in the meta-analysis. Thus, the present systematic review and meta-analysis aimed to compare the effects of nocturnal PCV and PSV on sleep quality in subjects who were critically ill.
Methods
Inclusion Criteria
The present meta-analysis examined randomized controlled trials and randomized crossover trials that (1) examined subjects receiving mechanical ventilation via endotracheal intubation; (2) compared nocturnal PCV with PSV; and (3) clearly reported subject inclusion and exclusion criteria, medical treatment regimens, the severity of symptoms, and a definition and evaluation of acute-on-chronic respiratory failure. Conference abstracts and trials that included duplicate subject cohorts or participants who were < 18 y; were receiving a vasopressive, sedative, or narcotic agent; or were comatose were excluded.
Search Strategy and Study Selection
Relevant trials published before November 2023 were identified from the PubMed, Embase, and Cochrane Library databases. The following key words and Medical Subject Headings were used in the search: “critically ill patients” OR “ICU patients” OR “mechanically ventilated patients” AND “pressure controlled ventilation” OR “assist-control ventilation” AND “pressure-support ventilation” AND “sleep quality” OR “sleep efficiency” OR “non-rapid eye movement” OR “rapid eye movement” OR “fragmentation index” OR “sleep fragmentation” OR “arousal” OR “awakening” OR “wakefulness.” No language restrictions were applied. Ongoing trials were searched for at the ClinicalTrials.gov registry. This meta-analysis was registered online at PROSPERO (International Prospective Register of Systematic Reviews), part of the National Institute for Health Research (registration CRD42023410731).
Data Extraction
Two of the authors (T-TC and M-CH) independently extracted baseline data and data with regard to outcomes, study design, study population characteristics, inclusion and exclusion criteria, duration of mechanical ventilation, course of respiratory failure, modes and settings of mechanical ventilation, and posttreatment parameters. The decisions of these two authors (K-WL and M-CH) were individually recorded and compared; disagreements concerning data extraction were resolved through discussion with a third reviewer (K-WT). The search strategy is summarized in the supplementary material (see the supplementary materials at http://www.rcjournal.com).
Methodological Quality Appraisal
Two reviewers independently assessed the methodological quality of each trial by using the Cochrane risk-of-bias tool for randomized trials.6 For crossover trials, we assessed bias that arises from the randomization process, intended intervention, missing outcome data, measurement of outcomes, and selection of reported results. In addition, we considered the period and carryover effects in the analysis. Disagreements with regard to risk-of-bias assessments were resolved through comprehensive discussion.
Outcomes
The primary outcome was sleep efficiency (ie, the percentage of time spent in sleep out of the total recording time). The secondary outcomes were wakefulness (ie, the percentage of time spent awake out of the total recording time), percentages of REM (rapid eye movement) sleep and stages 3 and 4 of non–REM sleep, the fragmentation index (ie, number of arousals and awakenings per hour of sleep), and the incidence of apneic events.
Statistical Analyses
We analyzed data in Review Manager version 5.4 (Cochrane Collaboration, Oxford, United Kingdom) and performed our literature review in accordance with the PRISMA guidelines.7 We calculated risk ratios for binary outcomes and mean differences for continuous outcomes with corresponding 95% CIs. SDs were estimated based on the provided CI limits or standard errors. If the mean and variance were not reported in a trial, then they were estimated based on the median, interquartile range, range, and sample size if the degree of skewness was acceptable. Efficacy and safety outcomes were analyzed by using a random-effects model.8 I2 statistics were calculated to evaluate the statistical heterogeneity of the trials, and the I2 value was used to quantify the proportion of total outcome variability across trials.6,9 The Cochran's Q test was used to determine the inconsistency of treatment effects across trials. Significance was indicated by P < .10 for the Cochran's Q tests.
Results
Trial Characteristics
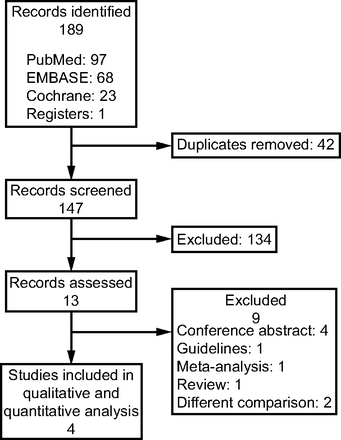
The study selection process is illustrated in Figure 1. The initial search yielded 189 articles. After the removal of duplicates, 147 studies remained, of which 134 were determined to be ineligible after the screening of their titles and abstracts. After the remaining 13 studies were reviewed, 9 studies were excluded (4 conference abstracts, 1 guideline, 1 meta-analysis, 1 review, and 2 studies with different comparisons). As a result, 4 randomized crossover trials were included in the final meta-analysis.10-13 The included trials were published between 2002 and 2013.10-13 Of these, 2 mainly enrolled subjects with COPD on mechanical ventilation10,13; one evaluated subjects who were critically ill and who were conscious, free from sedation and opiate analgesia, and receiving PSV with 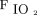 < 0.60% and
< 0.60% and 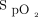 > 90%11; and one assessed 11 subjects receiving mechanical ventilation, of whom 6 were diagnosed with congestive heart failure.12 The age ranged from 65.4 to 70 years.10-13 The duration of mechanical ventilation ranged from 5.8 to 10 d in the 2 trials that mainly enrolled subjects with COPD10,13 and from 16.7 to 22 d in the other 2 trials, which examined subjects in the ICU.11,12 The indication for initial mechanical ventilation varied by trial. All of the included trials categorized their subjects into 2 groups: those receiving nocturnal PCV and those receiving conventional PSV.10-13 With regard to the settings for PCV, inspiratory pressure was set to 20 cm H2O to diminish spontaneous breathing in one trial,10 inspiratory pressure was provided to maintain a tidal volume (VT) of 8 mL/kg of predicted body weight in 2 trials,11,12 and inspiratory pressure was adjusted to obtain a VT of 10 mL/kg of predicted body weight in the remaining trial.13
> 90%11; and one assessed 11 subjects receiving mechanical ventilation, of whom 6 were diagnosed with congestive heart failure.12 The age ranged from 65.4 to 70 years.10-13 The duration of mechanical ventilation ranged from 5.8 to 10 d in the 2 trials that mainly enrolled subjects with COPD10,13 and from 16.7 to 22 d in the other 2 trials, which examined subjects in the ICU.11,12 The indication for initial mechanical ventilation varied by trial. All of the included trials categorized their subjects into 2 groups: those receiving nocturnal PCV and those receiving conventional PSV.10-13 With regard to the settings for PCV, inspiratory pressure was set to 20 cm H2O to diminish spontaneous breathing in one trial,10 inspiratory pressure was provided to maintain a tidal volume (VT) of 8 mL/kg of predicted body weight in 2 trials,11,12 and inspiratory pressure was adjusted to obtain a VT of 10 mL/kg of predicted body weight in the remaining trial.13
Flow chart.
With regard to the settings for PSV, inspiratory pressure of 6 cm H2O was provided to maintain SpO2 > 92% in 2 trials;10,13 inspiratory pressure was provided to maintain a VT of 6–8 mL/kg of predicted body weight (with a mean pressure support level of 16 cm H2O) or an automatically adjusted PSV instrument, namely a closed-loop system known as SmartCare (Dräger, Lübeck, Germany) was adopted to maintain a breathing frequency of 15–30 breaths/min, VT of >300 mL, and end-tidal CO2 of <55 mm Hg (with a mean pressure support level of 14 cm H2O) in one trial11; and inspiratory pressure was provided to obtain a VT of 8 mL/kg of predicted body weight (with mean pressure support levels of 16.8 cm H2O in subjects with central apnea and 19.6 cm H2O in subjects without central apnea) with or without the dead space (100 mL) in the remaining trial.12 The characteristics of the included studies are summarized in Table 1.
Characteristics of the Selected Randomized Crossover Trials
The methodological quality of the included studies is summarized in Table 2. With regard to allocation bias, one trial did not describe the allocation concealment method; however, the subjects’ baseline characteristics were balanced in this trial.12 With regard to attrition bias, 25.7% of the included subjects had missing outcome data in one trial.10 With regard to measurement bias, one trial did not describe the outcome assessment blinding strategy; however, in that trial, objective outcomes were not influenced by knowledge of the intervention.12 With regard to reporting bias, none of the trials provided registration information, but all relevant outcomes were reported.10-13 In addition, none of the trials provided information with regard to period or carryover effects.10-13 Overall, 3 trials were rated as having a low risk of bias,11-13 and another trial was rated as having some concerns with regard to risk of bias.10
Methodological Quality of the Included Crossover Trials
Sleep Efficiency
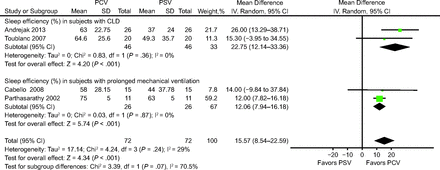
All of the analyzed trials assessed sleep efficiency (ie, the percentage of time spent in sleep of the total recording time).10-13 A neurophysiologist blinded to the assignment randomization determined sleep efficiency by using sleep electroencephalography.10,11,13 For the trial conducted by Cabello et al,11 we mainly compared the PCV group with the clinically adjusted PSV group. Overall, sleep efficiency was significantly higher in the PCV group than in the PSV group (mean difference 15.57%, 95% CI 8.54%–22.59%) (Fig. 2). Subgroup analyses of the influence of participant characteristics on sleep efficiency were performed; sleep efficiency was higher in the PCV group than in the PSV group (subjects with chronic lung disease: mean difference 22.75%, 95% CI 12.14%–33.36%10,13; subjects with prolonged mechanical ventilation: mean difference 12.06%, 95% CI 7.94%–16.18%11,12) (Fig. 2).
Effects of pressure controlled ventilation (PCV) vs pressure support ventilation (PSV) on sleep efficiency. CLD = chronic lung disease.
Wakefulness
Two of the analyzed trials assessed wakefulness (ie, the percentage of time spent awake out of the total recording time).10,13 Polysomnography recordings were used to determine wakefulness and were scored by a neurophysiologist blinded to the assignment randomization.10,13 Wakefulness was significantly lower in the PCV group than in the PSV group (mean difference −18.67%, 95% CI −30.29% to −7.04%) (Fig. 3).
Effects of pressure controlled ventilation (PCV) vs pressure support ventilation (PSV) on wakefulness.
Stages 3 and 4 Non-REM and REM Sleep
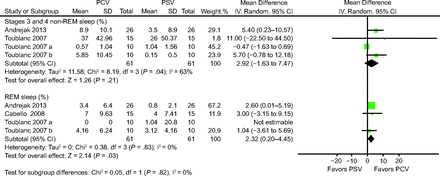
Three of the analyzed trials assessed the percentages of REM sleep and stages 3 and 4 non-REM.10,11,13 Electroencephalography recordings were used to measure the percentages of REM sleep and were analyzed by a neurophysiologist blinded to the assignment randomization process.10,11,13 The results of Cabello et al11 were mainly compared between the PCV group and the clinically adjusted PSV group. The results of Toublanc et al13 were examined in terms of the effects of PCV in 2 study periods (10 pm to 2 am and 2 to 6 am). The percentage of stages 3 and 4 non-REM sleep was higher in the PCV group than in the PSV group, but the difference was nonsignificant (mean difference 2.92%, 95% CI −1.63% to 7.47%) (Fig. 4). The percentage of REM sleep was significantly higher in the PCV group than in the PSV group (mean difference 2.32%, 95% CI 0.20%–4.45%) (Fig. 4). To determine whether the different study periods influenced our findings, we performed a sensitivity analysis to manage the high heterogeneity in this respect. Because the trial by Cabello et al11 was conducted partially in the daytime, we excluded the data from this trial. The sensitivity analysis revealed a similar result (mean difference 2.84%, 95% CI −1.97% to 7.65%), and the results indicated that the study periods (daytime and nighttime) did not influence the results. The results of sensitivity analysis are provided in the supplementary material (see the supplementary materials at http://www.rcjournal.com).
Effects of pressure controlled ventilation (PCV) vs pressure support ventilation (PSV) on rapid eye movement (REM) sleep and stages 3 and 4 non-REM (NREM) sleep.
Sleep Fragmentation Index
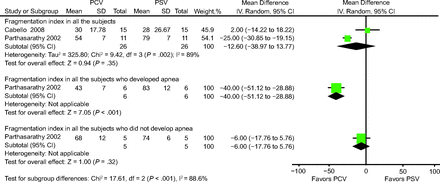
Two of the analyzed trials assessed the sleep fragmentation index (ie, the number of arousals and awakenings per hour of sleep).11,12 Polysomnography tracings were used to calculate the fragmentation index and were measured by a neurophysiologist blinded to the assignment randomization.11 Arousals were defined as a sudden shift in the electroencephalography frequency that comprised theta, alpha, and frequencies > 12 Hz and that lasted at least 3 s, which indicated wakefulness; awakenings were defined as electroencephalography recordings that were similar to those for wakefulness and lasted for more than half of a 30-s epoch in 2 trials.10-12 The results of Cabello et al11 were mainly compared between the PCV group and the clinically adjusted PSV group. The sleep fragmentation index was lower in the PCV group than in the PSV group (mean difference −12.60%, 95% CI −38.97% to 13.77%), but the difference was nonsignificant (Fig. 5). In the trial conducted by Parthasarathy and Tobin,12 among the 6 subjects with heart failure and who developed apnea, the sleep fragmentation index was significantly lower in those receiving PCV than in those receiving PSV (mean difference −40.00%, 95% CI −51.12% to −28.88%) (Fig. 5). By contrast, among the 5 subjects who did not develop apnea, the sleep fragmentation index did not significantly differ between the 2 groups (mean difference −6.00%, 95% CI −17.76% to 5.76%) (Fig. 5).
Effects of pressure controlled ventilation (PCV) versus pressure support ventilation (PSV) on the sleep fragmentation index.
Incidence of Apneic Events
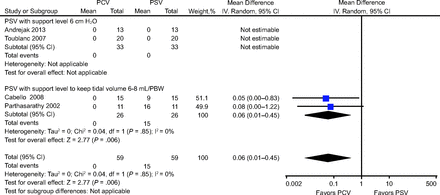
All of the analyzed trials assessed the incidence of apneic events.10-13 Polysomnography data were used to determine the incidence of apneic events and were recorded by a neurophysiologist blinded to the assignment randomization.10,11,13 Subgroup analyses were performed to examine the influence of subject characteristics on the incidence of apneic events. No apnea was recorded during PSV or PCV in 2 trials.10,13 Rather than obstructive apnea, central apnea occurred in 9 of 15 subjects in the trial by Cabello et al11 and in 6 of 11 subjects in the trial by Parthasarathy and Tobin.12 The incidence of apneic events was significantly lower in the PCV group than in the PSV group (risk ratio 0.06, 95% CI 0.01–0.45) (Fig. 6).
Effects of pressure controlled ventilation (PCV) vs pressure support ventilation (PSV) on the incidence of apneic events. PBW = predicted body weight.
Discussion
Our findings indicated that nocturnal PCV improved sleep efficiency, reduced wakefulness, inhibited apnea, extended REM sleep, and prevented arousals and awakenings during sleep in subjects who were likely to develop central apnea. Therefore, nocturnal PCV seems to be useful for improving sleep efficiency in patients who are clinically ill, especially those with COPD or congestive heart failure and those who require prolonged mechanical ventilation. Effective mechanical ventilation strategies must be implemented in patients who are critically ill with chronic lung disease because such patients are particularly susceptible to respiratory deficits. When implementing such strategies, diminished endurance of the respiratory muscles is a key factor that must be considered.14 Patients with respiratory failure often have difficulty weaning off mechanical ventilation. In the trial conducted by Andréjak et al,10 the percentage of stages 3 and 4 non-REM sleep was higher in subjects receiving nocturnal PCV than in those receiving PSV, which indicates that respiratory muscle endurance was positively correlated with sleep quality in that study. According to that finding, nocturnal PCV may relieve respiratory demand, and, in PCV, the respiratory muscles are at rest.
Respiratory muscle endurance may influence sleep quality.4,14 In the trials conducted by Andréjak et al10 and Cabello et al,11 the subjects receiving nocturnal PSV experienced more sleep disturbances than did those receiving nocturnal PCV, although the difference was nonsignificant in the trial by Cabello et al.11 The only difference between these 2 studies was their inclusion criteria. Andréjak et al10 primarily included subjects with COPD, whereas Cabello et al11 enrolled no subjects with chronic lung diseases. Patients with COPD or respiratory failure have impaired respiratory muscle endurance and difficulty being weaned off mechanical ventilation.10,13 Accordingly, the use of nocturnal PCV in patients with impaired respiratory muscle endurance may be beneficial.
Compared with PSV, nocturnal PCV has several advantages. First, in nocturnal PCV, the respiratory muscles are at rest; this feature is especially useful during the weaning process. Second, nocturnal PCV improves sleep efficiency and reduces wakefulness in patients with COPD or congestive heart failure.10,12,13 Such patients tend to develop sleep apnea.12 Such sleep disruption may impair inspiratory muscle endurance and can lead to hypercapnia.14,15 The pressure-time index was found to be lower in patients with sleep deprivation.14 Furthermore, in one study, the strength of hypoxic and hypercapnic ventilatory responses decreased after a single night without sleep.15 Accordingly, nocturnal PCV may play a role in decreasing the duration of mechanical ventilation in patients with impaired respiratory muscle endurance.14 Third, sleep-promoting interventions have been associated with shorter durations of delirium.4,16 Therefore, sleep-promoting interventions, especially those related to ventilatory modes, may be beneficial and thus warrant further evaluation.
The support level of PSV should be considered by clinicians and respiratory therapists.4,10,13 In our meta-analysis, the incidence of central apnea in subjects receiving PSV was significantly higher in the trials conducted by Cabello et al11 and Parthasarathy and Tobin12 than in those conducted by Andréjak et al10 and Toublanc et al.13 The difference in the incidence of central apnea among these trials may be attributable to the pressure support level used in each trial. Andréjak et al10 and Toublanc et al13 mainly set the pressure level at 6 cm H2O to overcome the resistance of the endotracheal tube. By contrast, Cabello et al11 set the pressure support level, which was reduced, on the basis of each subject’s condition (with a mean pressure support level of 15 cm H2O), whereas Parthasarathy and Tobin12 adjusted the pressure support level to maintain a VT at 8 mL/kg of predicted body weight (with mean pressure support levels of 16.8 cm H2O in subjects with central apnea and 19.6 cm H2O in subjects without central apnea). Excessive pressure may induce apneic events in patients during the weaning process.
Our meta-analysis has several limitations. First, the sample sizes of all the included trials were small; therefore, the present findings may be inconclusive. Second, the level of noise pollution experienced by the subjects in the included trials is likely to have been lower than that experienced by patients in real-life clinical settings, which may have underestimated the effect sizes in our meta-analysis. Third, variations in the inclusion criteria and ventilator type may have caused heterogeneity among the trials; however, we accounted for these variations by performing subgroup analyses. Fourth, the included trials were conducted 10–20 years ago; thus, advancements in medical technology may influence the results if these trials were to be replicated now. Fifth, although several related studies revealed that sleep fragmentation is associated with cardiovascular morbidity,17,18 we found no relevant outcome data, such as those related to the length of ICU length of stay, morbidity, or mortality, in our included trials.10-13 Sixth, because information with regard to the type of sedation during ventilation support was not available, we could not clarify the association between sedative agents and sleep quality.10-13 Hence, the effects of nocturnal PCV on patients receiving mechanical ventilation should be further investigated in future studies.
Conclusions
The present meta-analysis revealed that nocturnal PCV improved sleep quality in subjects receiving mechanical ventilation, especially those with impaired respiratory muscle endurance or a tendency to develop apnea.
Footnotes
- Correspondence: Ming-Chi Hu RRT, Department of Pulmonary Medicine, Shuang Ho Hospital, Taipei Medical University, No. 291, Zhongzheng Rd., Zhonghe Dist., New Taipei City, 23561, Taiwan. E-mail: 20549{at}s.tmu.edu.tw
The authors have disclosed no conflicts of interest.
This work was primarily performed at the Respiratory Therapy Room, Division of Pulmonary Medicine, Department of Internal Pulmonary Medicine, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan.
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
This manuscript was edited by Wallace Academic Editing.
Supplementary material related to this paper is available at http://rc.rcjournal.com.
- Copyright © 2024 by Daedalus Enterprises