Abstract
The use of ventilatory assistance can be traced back to biblical times. However, mechanical ventilators, in the form of negative-pressure ventilation, first appeared in the early 1800s. Positive-pressure devices started to become available around 1900 and today's typical intensive care unit (ICU) ventilator did not begin to be developed until the 1940s. From the original 1940s ventilators until today, 4 distinct generations of ICU ventilators have existed, each with features different from that of the previous generation. All of the advancements in ICU ventilator design over these generations provide the basis for speculation on the future. ICU ventilators of the future will be able to integrate electronically with other bedside technology; they will be able to effectively ventilate all patients in all settings, invasively and noninvasively; ventilator management protocols will be incorporated into the basic operation of the ventilator; organized information will be presented instead of rows of unrelated data; alarm systems will be smart; closed-loop control will be present on most aspects of ventilatory support; and decision support will be available. The key term that will be used to identify these future ventilators will be smart!
Introduction
The refinement and continual development as well as the expanded clinical application of the mechanical ventilator have been prominent factors in the development and growth of the profession of respiratory care as well as critical care medicine. The need for mechanical ventilation is a common feature of the patient requiring admission to the intensive care unit (ICU). Indeed, the expansion and increased sophistication of the mechanical ventilator parallels similar developments in the respiratory therapy profession.
In this review the historical development of mechanical ventilators, positive and negative pressure, invasive and noninvasive will be discussed. The goal will be to try to identify the changes in clinical medicine that prompted refinement of the mechanical ventilator. The generational development of positive-pressure ICU ventilators will be addressed, as will the capabilities of today's ICU ventilators. This will all provide the background for speculation on the ICU ventilator of the future.
Negative-Pressure Ventilators
Throughout the 19th century and the first half of the 20th century the negative-pressure ventilator was the predominant device used to provide ventilatory assistance. The first description of a negative-pressure ventilator was of a full-body type ventilator. This “tank ventilator” was first described by the Scottish physician John Dalziel in 1838.1 It consisted of an air-tight box, with the patient maintained in the sitting position. Negative pressure was established by manually pumping air into and out of the box (Fig. 1). The device was equipped with a pressure gauge to monitor the extent of negative pressure established in the device. A number of other groups developed similar types of manually operated negative-pressure ventilators.2 In 1904 Sauerbrach even developed a negative-pressure operating chamber (Fig. 2).3 The patient's body, except for the head, was maintained inside the chamber. The chamber was large enough so that the surgeon was able to perform surgery while also in the chamber. The patient's lower body was encased in a flexible sack so that positive pressure could be applied to this part of the body, preventing blood from accumulating in the abdomen and lower extremities, causing what was referred to as “tank shock.”4
Negative-pressure operating chamber. (From Reference 3, with permission.)
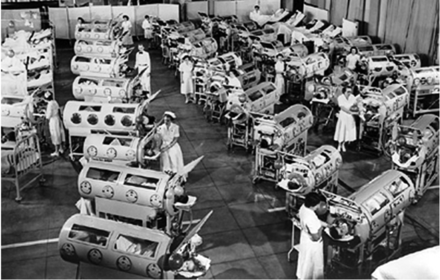
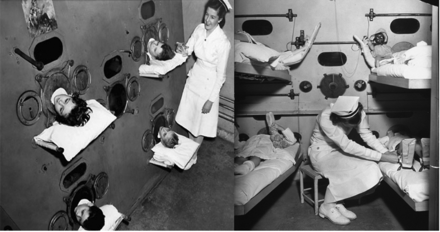
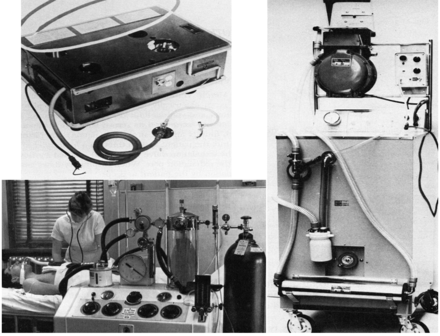
Negative-pressure ventilation became a much greater clinical reality with the development of the iron lung, originally designed and built by Drinker and Shaw,5 but manufactured and sold by Emerson.6 This approach to ventilatory support reached its pinnacle during the worldwide poliomyelitis epidemics from 1930 to 1960.7 The first ICUs were set up to manage in some cases dozens of patients, of all ages, requiring negative-pressure ventilation because of poliomyelitis (Fig. 3).8 Boston Children's Hospital developed a large negative-pressure chamber that could accommodate 4 children simultaneously and allow a nurse to care for the patients from inside the chamber (Fig. 4).8
Poliomyelitis epidemic patients at Ranchos Los Amigos Hospital, California, 1953. (From Reference 8.)
Multi-person negative-pressure ventilator at Boston Children's Hospital, 1950s. (From Children's Hospital Boston Archives, with permission.)
Over time, numerous other types of negative-pressure chambers were developed and used, with varying success, such as the “raincoat” and the “chest cuirass” (Fig. 5).6,9 However, in the 1960s there was a movement away from negative-pressure ventilation because of several factors. The first volume-targeted ICU/anesthesia ventilators began to appear. Second, the development of jet aviation at the close of the second world war lead to the development of small, compact, intermittent positive-pressure breathing (IPPB) devices: the Bennett and Bird IPPB machines (Fig. 6).10,11 Third, problems with the application of negative-pressure ventilation became too much for their continued use in the newly developing ICUs. As a group, these devices were large, heavy, and cumbersome, and it was difficult to avoid excessive leaking (generally resulting in cooling of the patient's body); they had a difficult time maintaining effective ventilation, were unable to sustain high airway pressure or establish PEEP, access to the patient was limited, and “tank shock” was an ongoing issue with full-body ventilators.9
Left: Chest cuirass (“turtle shell”), Right: “Raincoat” wrap with wire grid and Emerson 33-CRE negative-pressure ventilator. (From Reference 9.)
Left: Bird Mark 7 and Bird Mark 8. Right: Bennett TV-2P and Bennett PR-2.
Positive-Pressure Noninvasive Ventilation
Positive-pressure noninvasive ventilation (NIV) can be traced back to biblical times: 2 specific passages from the Bible reference NIV4:
And the Lord God formed man of the dust of the ground and breathed into his nostrils the breath of life.
(Genesis 2:7)
And he [Elisha] went up, and lay upon the child, and put his mouth upon his mouth and the flesh of the child waxed warm.
(II Kings 4:34)
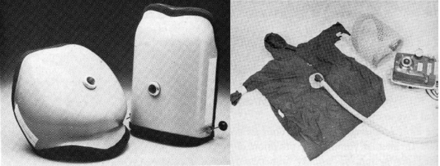
However, the first mechanical apparatus used to provide NIV, a bag and mask manual ventilator, was introduced in 1780 by Chaussier12 (Fig. 7). A more sophisticated bellows with a mask was introduced in 1887 by Fell12 (see Fig. 7), and in 1911 Dräger's Pulmotor was first introduced.12 This was a fairly sophisticated pneumatically operated positive-pressure device that has been credited with saving thousands of individuals over its lifetime (see Fig. 7).12 Another approach to providing NIV was introduced by Green and Janeway in 1910 (Fig. 8).12 They referred to their device as a “rhythmic inflation apparatus.” The patient's head was placed into the apparatus and a seal was secured around the patient's neck with positive pressure applied to the patient's head. However, the most notable NIV devices of the 20th century were the Bennett TV and PR series and the Bird Mark series of ventilators (see Fig. 6).11 These devices were used primarily to provide intermittent treatments, as opposed to long-term ventilation, but in the 1960s and 1970s it was common to see them used for life support in both noninvasive and invasive ventilation.
Dräger Pulmotor. (From Reference 12, with permission.)
Green and Janeway rhythmic inflation apparatus, 1910. (From Reference 12, with permission.)
During the late 1970s and early 1980s, two things happened that changed the concept of NIV. First the IPPB machine faded from use as a result of reports that the use of the IPPB machine to deliver aerosolized medication was no better than a simple nebulizer13,14 and that incentive spirometer15 and blow bottle16 were as good as IPPB in preventing and reversing postoperative atelectasis. The second event was a series of case studies indicating that NIV could be used to provide ventilatory support to patients in an exacerbation of chronic lung or neuromuscular/neurologic disease, or to provide long-term ventilator support to those same patients.17–20 In general, this type of support was first provided with volume control modes capable of only machine-triggered inspiration (Fig. 9).21 But over time newer more sophisticated pressure-targeted ventilators (Fig. 10) designed specifically to provide NIV entered the market, and pressure-targeted ventilation became the norm for NIV. Today, NIV modes have become available on most new ventilators entering the market, and NIV22,23 has become the standard for initial ventilatory support for numerous pathophysiological conditions.6
Noninvasive ventilation with a ventilator providing only volume control without patient triggering. (From Reference 21.)
Current commercially available noninvasive ventilators. Clockwise from upper left: Respironics STD30, Respironics V60, Dräger Carina, Respironics Vision, Sullivan VPAP.
Positive-Pressure Invasive Ventilators
First-Generation ICU Ventilators
Ventilators designed for positive-pressure invasive ventilation became available in the 1940s and 1950s. Figure 11 shows some of the early models. The key distinguishing feature of these early invasive ventilators was that they provided only volume-control ventilation (Table 1). Patient-triggered ventilation was not possible with these first-generation ICU ventilators.11,12 However, the range of sophistication of these ventilators was quite large. The Morch ventilator was a single-circuit, simple, piston ventilator. It was one of the least complex of this group and was designed to be placed under the patient's bed (see Fig. 11). This ventilator had no monitors, no alarms, and no specific setting. The respiratory rate had to be counted and the tidal volume measured with a secondary device. Gas was always delivered at an inspiratory/expiratory ratio of 1:2.12 On the other end of the spectrum was the Engstrom ventilator, which, because it had a double-circuit, could be used as an anesthesia machine or as an ICU ventilator.12 Although its monitoring capabilities were limited by today's standards, it did include airway pressure and tidal volume monitoring and allowed for more exact setting of respiratory rate, but it still provided only machine-triggered inspiration at a 1:2 inspiratory/expiratory ratio. The Emerson postoperative ventilator was between those 2 extremes. It was also a volume-controlled ventilator and provided only machine-triggered inspiration, but it had an adjustable inspiratory/expiratory ratio and pressure and volume monitoring. But it could not be used for anesthetic gas delivery because it had only a single circuit.12
Ventilators providing volume control only without patient triggering. Clockwise from upper left: Morch ventilator, Emerson postoperative ventilator, Engstrom ventilator. (From Reference 12, with permission.)
Generations of Intensive Care Ventilators
This first generation of ICU ventilators did not incorporate PEEP. It was not until after the landmark paper by Ashbaugh et al24 that PEEP became a standard therapy in the ICU. With this generation of ventilators, PEEP was applied as shown in Figure 12,25 by placing the expiratory limb of the circuit under water, at a depth equal to the desired PEEP. This generation of ventilators ended in the early 1970s with the introduction of the Puritan Bennett MA-1 ventilator.
First approach to the application of PEEP. (From Reference 25.)
Second-Generation ICU Ventilators
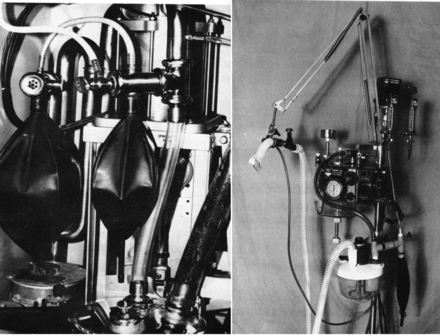
The second generation of ICU ventilators differed from the first in a number of ways. Simple patient monitors were incorporated into the ventilator itself. Most monitored tidal volume and respiratory rate, but the most distinguishing feature of this generation of ventilators was patient-triggered inspiration. But still only volume ventilation was available.26 This also is the first group of ventilators that included basic alarms such as high pressure, high rate, and low tidal volume. Soon after the introduction of this generation of ventilators, intermittent mandatory ventilation (IMV) was introduced into adult ventilation. Downs et al published the first case series using IMV in 1973.27 They used an external secondary IMV gas flow system introduced into the ventilator circuit (Fig. 13).28 Later ventilators of this generation added demand values, and IMV became synchronized intermittent mandatory ventilation (SIMV).29 In addition to the MA-1, the Siemens Servo and Ohio 560 ventilators were typical ventilators of this generation (Fig. 14). The introduction of the Servo 900C at the end of this generation introduced into clinical practice pressure-support and pressure-control ventilation.30
Original intermittent mandatory ventilation setups for the Emerson postoperative ventilator and the Bird Mark 14 ventilator. (From Reference 28.)
Clockwise from upper left: Puritan Bennett MA-1, Ohio 560, Siemens Servo 900.
In the late 1970s a publication by Hewlett et al provided a glimpse into the future of ventilator modes.31 As illustrated in Figure 15, they were the first to demonstrate the concept of closed-loop ventilation. Although their approach to mandatory minute ventilation was purely mechanical, it did function as a closed-loop controller and provided a model for many of the modes of today. Gas entered this system (see Fig. 15) at the left and preferentially entered a bellows from which the patient could breathe spontaneously. If the bellows filled completely, gas was directed to a second bellows. Once that bellows filled, gas from the bellows was delivered to the patient as a positive-pressure breath. Dependent on the flow of gas into the system, the setting of the bellows capacity, and the patient's spontaneous minute volume, all breaths were either spontaneous or mandatory (if the patient became apneic) or a mix of the two. The primary problem with this initial system was that the patient could breathe the entire minute volume with a very rapid and shallow breathing pattern, but it did provide the first form of closed-loop control.
The first mandatory minute ventilation system (see text). (From Reference 31, with permission.)
Third-Generation ICU Ventilators
Typical third-generation ICU ventilators were the Puritan Bennett 7200, the Bear 1000, the Servo 300, and the Hamilton Veolar (Fig. 16). The single most important factor that all of these ventilators had in common was microprocessor control. This was a major event in the development of mechanical ventilators, because it meant that virtually any approach to gas delivery and monitoring was possible. All that was required was innovation, engineering skill, and money! In addition, mechanisms for gas delivery were vastly enhanced. These ventilators were markedly more responsive to patient demand than any of the previous generations of mechanical ventilators.32 Flow-triggering also became a reality, again reducing the effort patients needed to activate gas delivery. Almost every ventilator of this era included pressure support, pressure control, volume control, and SIMV. SIMV was not only available in volume ventilation, but also pressure ventilation and pressure support could be applied during the spontaneous breaths.33
Puritan Bennett 7200.
All of the ventilators of this generation also incorporated extensive alarms and monitors. They not only monitored the patient's status, but almost every aspect of the ventilator's functioning. This was also the generation of ventilators in which waveforms of pressure, flow, and volume were first introduced, along with pressure-volume and flow-volume loops.31
With this generation of ventilators was the first use of airway pressure release ventilation, by Stock et al.34 The circuit used by Stock et al (Fig. 17) was a simple high-flow system that incorporated a solenoid valve and 2 PEEP valves. The approach applied high levels of continuous positive airway pressure (CPAP) but periodically reduced the CPAP to a lower level to assist with ventilation. The solenoid could be programmed to apply any ratio of inspiratory and expiratory time between high and low CPAP, as well as any frequency of dropping to the low CPAP level.
The first application of airway pressure release ventilation. (From Reference 34, with permission.)
Fourth-Generation ICU Ventilators
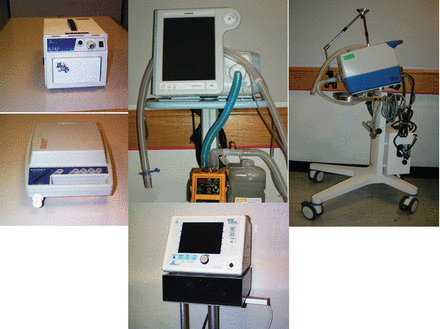
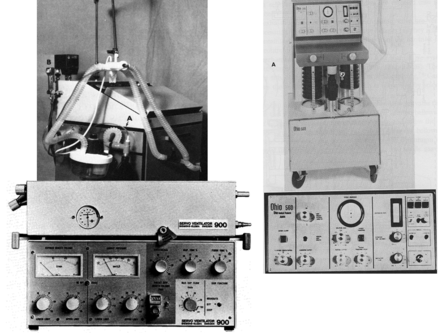
This is the current generation of ICU ventilators, which are the most complex and versatile of any mechanical ventilators ever manufactured (Fig. 18). In this era there has clearly been a marked increase in the number of ventilators, of all possible types. Numerous ventilators classified as ICU ventilator are available worldwide. There are a number of what have been referred to as sub-acute ventilators, as well as transport/home-care ventilators and ventilators designed specifically for NIV applications.
Current-generation intensive care unit ventilators. Left to right: Covidien/Puritan Bennett 840, CareFusion Avea, Maquet Servo-i.
The single feature that distinguishes this generation is the plethora of ventilation modes available. In addition, many of these new modes are based on closed-loop control. The question that we all should be asking manufacturers regarding these new modes is, “Do they provide value, or are they simply more bells and whistles?” The questions I use to determine if a new ventilation mode is useful are:
Does it make ventilation safer?
Does it decrease the likelihood of ventilator-induced lung injury or hemodynamic compromise?
Does it more effectively ventilate or oxygenate the patient?
Does it wean the patient from ventilatory support faster?
Does it improve patient-ventilator synchrony?
If the answer to each of those questions is no, then the mode is essentially useless. Fortunately, most of these newer modes do seem to have a yes answer to at least one of the questions.
Most of these new modes are, for the most part, based on a pressure-targeted approach. Maybe the most complex of these modes is adaptive support ventilation, which attempts to establish a ventilatory pattern based on the Otis work-of-breathing model.35 The clinician enters the patient's ideal body weight, desired minute volume, and maximum airway pressure, and the ventilator determines the respiratory rate and tidal volume combination that results in the least work of breathing. This pattern is established by the ventilator automatically adjusting the ventilating pressure and respiratory rate.36 In the newest version of this mode, end-tidal PCO2 is also added as an input.37 Initial data suggest that this mode works well in some patients.38,39 But, like all of these modes, additional research is needed to identify when it should be used.
SmartCare is another form of closed-loop control of pressure support for weaning.40 The ventilator automatically adjusts the pressure support level every 2–4 min to maintain a predefined respiratory rate, tidal volume, and end-tidal PCO2, with separate algorithms for COPD patients, for use with endotracheal tubes versus tracheostomy tubes, and for those with active versus passive humidification. When the pressure support level is reduced to a predetermined level, the ventilator automatically performs a spontaneous breathing trial (SBT).41 If the patient fails the SBT, the ventilator automatically resumes ventilation. If the patient passes the SBT, the ventilator notifies the user that the patient should be considered for extubation. A recent randomized controlled trial compared SmartCare to clinician-performed weaning and found that patients were weaned faster with SmartCare.40 The study has been criticized because in the control arm the clinician-applied SBTs were not consistently performed, being missed 50% of the time. A more recent study in which the control group was weaned per the protocol, found no benefit from SmartCare.42 However, in the real world, modes of this type are very useful, because they ensure that when the patient meets defined criteria, the appropriate care is provided regardless of how busy the clinician may be.
Proportional assist ventilation and neurally adjusted ventilatory assist are available on the fourth generation of ventilators, but should be considered modes of the future.43 With both of these modes, pressure, flow, volume, and time are not set. What is set is the proportion of a patient's ventilatory effort that is unloaded without forcing a ventilatory pattern. Proportional assist ventilation functions by responding to the mechanical output of the diaphragm and accessory muscles of inspiration (inspiratory flow and volume),44 whereas neurally adjusted ventilatory assist functions by responding to the neural input to the diaphragm (electrical activity).45 Data from physiologic studies of these modes indicate that, when patients are transitioned to either mode, synchrony improves, tidal volume decreases, respiratory rate increases, and peak airway pressure decreases,46–51 without adverse effects on gas exchange or hemodynamics. No randomized trials comparing these modes to conventional mechanical ventilation have been published to date, but I expect that the ability of these modes to improve patient outcomes will be shown in the future.
Almost all of the ventilators in this generation include NIV modes,22,23 and many are capable of ventilating neonates as well as adults.52 Currently the capability of the NIV modes on these ventilators varies widely. Some do a good job of compensating for leaks, whereas others do not. However, I predict that all of these ventilators will eventually provide NIV with the same efficacy as the ventilators designed specifically to provide NIV. As shown by Marchese et al,52 most of these ventilators are at least as capable of meeting the demands of neonates as a traditional neonatal ventilator. I expect that their function at this level will improve over time.
All of the ventilators of this generation are easily upgradable, include waveforms as a basic operating feature, and provide extensive monitoring. Each of them provides monitoring data of 20 to 40 individual variables. Many provide multiple screens of data presentation. Almost every possible patient and ventilator variable is displayed. Trending data are also available on most of these units.
Some of these ventilators include specific management/assessment packages. Some allow the clinician to program the performance of a pressure-volume loop. Others have programs that make it easy to perform recruitment maneuvers or decremental PEEP trials. Others have options that facilitate assessment for weaning and performance of weaning trials, whereas others allow for measurement of esophageal pressure and functional residual capacity. The current generation of ICU ventilators is far ahead of the ICU ventilators we used in the 1960s or 1970s. Considering how much change has occurred in ICU ventilators over the last 50 years, one can speculate on the future of ICU ventilators.
The Future ICU Ventilator
The ICU ventilator of the future may not look very different from today, but several features will clearly separate them from the current generation of ventilators (Table 2). There will be integration with other bedside technology. Within a few years, all ICUs will have electronic charting, where data from all bedside technology will be transmitted to electronic documentation systems. As a result, ventilators must be able to be integrated electronically with all other bedside technology.
Features of the Ventilator of the Future
The days of specific ventilators designed to do specific tasks such as neonatal ventilation, adult ventilation, NIV, and transport will be gone. The ICU ventilator of tomorrow will be able to perform all of these tasks as well or better than the individual ventilators of the past. The available evidence indicates that some ventilators are already capable of providing ventilation under multiple situations, and in the future all will.22,23,52
Protocols will become part of the basic operation of the ICU ventilator. As more evidence becomes available on how we should provide lung-protective ventilation, and on how we should manage specific diseases, ventilators will be able to integrate evidence-based algorithms into their basic operational approach. We should be setting tidal volume based on the patient's predicted body weight. The ventilator of the future will require us to input the patient's height and sex, and volumes will be presented as mL/kg predicted body weight, in addition to absolute volume. The Acute Respiratory Distress Syndrome Network53 protocol, as well as different approaches to performing lung-recruitment maneuvers54 and setting PEEP,55 will be selectable options on future ventilators. These approaches will still require the clinician to set basic parameters, but the ventilator will provide guidance to assure that ventilation for a specific disease state is performed within the current best evidence-based guidelines.
Much of the noise pollution in the ICU is a result of alarms. However, in the vast majority of circumstances the alarms are false. As a result, staff are programmed to ignore alarms (“alarm fatigue”). The ventilator of the future will correct this. Smart alarms will replace our current systems. For example, the high-pressure alarm does not need to sound every time pressure exceeds the set level. The ventilator of tomorrow will be able to identify the pattern of alarms.
We would interpret the following 3 scenarios differently, and there is no reason why the ventilator could not do the same thing. First, a periodic increase in airway pressure that exceeds the set level on an occasional basis. Second, a slowly increasing peak pressure over a number of hours with the tidal volume unchanged. Third, airway pressure increasing with each breath and the delivered tidal volume, once the limit is met, getting smaller with each breath. All 3 of these scenarios represent potential clinical situations with different levels of urgency in their response.
The first is most likely a result of secretions in the patient's airway or water in the ventilator circuit that periodically causes the peak airway pressure to rise. This is not an emergency. The second situation depicts a change in the patient's lung mechanics, requiring the clinician to determine the cause and potentially to adjust the ventilation approach. But this is not an emergency. The third scenario, however, is an emergency. This potentially indicates that a tension pneumothorax has developed and requires immediate response. Clearly the alarm conditions in these 3 scenarios should be markedly different. The ventilator of tomorrow will be able to interpret these patterns and the alarm conditions will be different.
The ventilators of tomorrow will not present lists and lists of unrelated data that are of little use to the clinician. An individual can process only finite individual pieces of data. The next generation of ventilators will present information: not merely lines of data. Easy to interpret graphs or figures will be displayed that allow the clinician to rapidly determine if the patient's status has changed. The use of a figure to represent a change in patient's status is already being used on at least one ventilator.56 Important interrelated variables will be presented so the clinician can rapidly determine if change has occurred. For example, tidal volume and airway pressure will be presented in a manner that the trends in these variables can be easily understood. In addition, information that traditionally has not been presented will be provided. The asynchrony index will be calculated and displayed, along with the number of breaths with missed triggers, with double-triggers, or that have an exceptionally short or long inspiratory or expiratory time. The presence of conditions that are associated with the development of auto-PEEP will be identified and displayed.
The most important thing that this new generation of ventilators will do is provide decision support. Each alarm condition will be followed with a listing of potential causes and potential solutions. Changes in ventilator variables will be identified and the clinician notified of the change, the potential causes, and the possible solutions. A library of information will be accessible from the ventilator screen, ranging from the ventilator's operation manual to the evidence that supports a recommended action.
Closed-loop control of ventilation will be available on all ventilation modes. These new ventilators will be able to adjust gas delivery to improve patient-ventilator synchrony. They will be able to interpret the airway pressure and flow waveform during both volume and pressure ventilation, and to automatically adjust the flow waveform, peak inspiratory flow, rise time, and termination criteria to ensure that gas delivery is synchronous with the patient's desires. This is an increasingly important factor in ventilator functioning, because we are finding out that patient outcome may be markedly affected by asynchrony.57,58 Automatic adjustment of termination criteria is already available on at least one ventilator.59
All of these expected changes mean that the users of the mechanical ventilators of the future will have to be even better prepared than the users of today. They will have to understand in detail the operational complexities of the new features. They will have to be able to determine when one feature is indicated over the other. They will have to make sure that the ventilator is truly doing what it is expected to do and that the patient is responding as expected to the intervention. The clinicians managing these patient-ventilator systems of the future will need to be much more capable than the current group of operators.
The historical development of the mechanical ventilator is truly a remarkable journey. In just 50 short years we have gone from relatively crude, totally mechanical devices that could provide only machine-triggered volume ventilation to highly evolved microprocessor controlled systems capable of any form of ventilatory support imaginable. The evolution of the mechanical ventilator mirrors the evolution of the profession of respiratory care as well as critical care medicine, and may even be the primary reason that respiratory care has grown to its current status. Finally, the single most descriptive term that will be used to define the future generation of mechanical ventilators will be smart.
What we learn from academic studies is knowledge: what we learn from experience is wisdom.
—Mohandas Gandhi
Footnotes
- Correspondence: Robert M Kacmarek PhD RRT FAARC, Respiratory Care, Warren 1225, Massachusetts General Hospital, 55 Fruit Street, Boston MA 01460. E-mail: rkacmarek{at}partners.org.
-
Dr Kacmarek presented a version of this paper as the 37th Donald F Egan Scientific Memorial Lecture at the 56th International Respiratory Congress of the American Association for Respiratory Care, held December 6–9, 2010, in Las Vegas, Nevada.
-
Dr Kacmarek has disclosed relationships with Covidien, Hamilton, Maquet, Kimberly-Clark, Newport, KCI, and Bayer.
- Copyright © 2011 by Daedalus Enterprises Inc.