Abstract
Turbuhaler and Diskus are commonly used powder inhaler devices for patients with respiratory disease. Their effectiveness is limited in part by a patient's ability to use them correctly. This has led to numerous studies being conducted over the last decade to assess the correct use of these devices by patients and health care professionals. These studies have generally used device-specific checklists to assess technique, this being the most feasible and accessible method for assessment. However, divergence between the checklists and scoring systems for the same device in different studies makes direct comparison of results difficult and at times inappropriate. Little evidence is available to assess the relative importance of different criteria; however, brief patient training based on specific inhaler technique checklists leads to significant improvement in asthma outcomes. This paper reviews common checklists and scoring systems used for Turbuhaler and Diskus, discusses the problem of heterogeneity between different checklists, and finally recommends suitable checklists and scoring systems for these devices based on the literature and previous findings. Only when similar checklists are used across different research studies will accurate comparisons and meta-analysis be possible.
Introduction
Powder inhaler devices have been developed over the last 2 decades for treatment of asthma and COPD. These small portable devices contain micronised powdered medication, often with a carrier, with the particles dispersed during inhalation. These devices were developed for use in adults and older children and have been shown to deliver drugs safely and effectively.1,2 Several types of powder inhalers are available, some as single-dose, capsule-based inhalers, such as the Aerolizer (Novartis, Basel, Switzerland), or HandiHaler (Boehringer Ingelheim, Ingelheim, Germany). More commonly, powder inhalers are multidose inhalers, either reservoir-type inhalers, such as the Turbuhaler (AstraZeneca, London, United Kingdom) (similar to the Flexhaler), Clickhaler (Vectura, Wiltshire, United Kingdom), Easyhaler (Orion, Espoo, Finland), and Novolizer (Meda, Solna, Sweden), or based on prefilled blisters, such as the Diskus Accuhaler (GlaxoSmithKline, Brentford, England), or blister disks, such as the Diskhaler (GlaxoSmithKline, Brentford, England).
The acceptance of powder inhalers has been driven in large part by the enormous success in recent years of combination treatment with a corticosteroid and a long-acting β2 agonist.3 The combination of budesonide and formoterol fumarate dihydrate (Symbicort) in the Turbuhaler and fluticasone propionate and salmeterol xinafoate (Seretide) in the Diskus reflects current treatment guidelines for moderate to severe asthma and COPD. These devices have been found to be the most efficient and commonly used of the powder inhaler devices.4,5
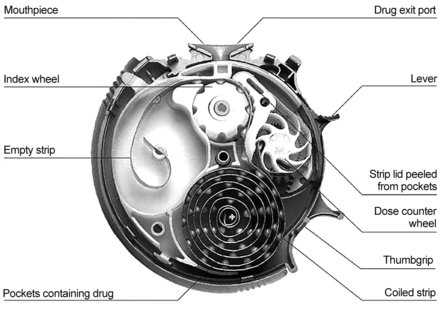
Many factors play a role in the overall performance of the Turbuhaler6 (Fig. 1) and Diskus7 (Fig. 2), from their pharmaceutical formulations to their mechanical and aerodynamic properties and to the way they are used by people with airway disease. Correct device use is critical to the delivery of medication to the airways. Many studies have been conducted to assess and improve the way people use the Turbuhaler and Diskus. Some studies have used specific devices for assessing individual components of inhaler technique. For example, devices such as the Turbutest measure the patient's inspiratory flow through a placebo Turbuhaler.8,9 Portable devices for assessing some components of inhaler technique were recently reviewed.10 One example is the Inhalation Manager, which measures inspiratory flow through inhalation devices (including a stylized powder inhaler) using a pneumotachometer; this system also predicts drug delivery mass and particle deposition based on in vitro data.11 This instrument is proposed as a tool for assessing and training patients on correct inhaler technique and for identifying the most suitable inhaler for an individual patient,11 but it is not feasible on a wide scale and requires technical support. The Turbuhaler whistle (AstraZeneca Pharmaceuticals) is a simple device that whistles if the patient inhales > 35 L/s.12
Turbuhaler. Courtesy AstraZeneca.
Diskus. Courtesy GlaxoSmithKline.
However, by far the most common, feasible, and accessible method of assessing inhaler technique is through the use of device-specific inhaler technique checklists. The validity of inhaler technique checklists was initially established by Appel,13 who showed that trained personnel were able to achieve a 98% success rate in predicting the bronchodilator response for patients with asthma using their reliever devices by observing their inhaler technique. More recently, inhaler technique checklists have been shown to be feasible tools for assessment of technique. The clinical utility of inhaler checklists has been confirmed by studies with powder inhalers14 and pressurized metered-dose inhalers,15 which have shown that, when brief patient training was based on a standardized inhaler technique checklist, there were significant improvements in asthma outcomes. Hence, it can be concluded that assessment and correction of asthma patients' inhaler technique against a checklist are valid measurements of the effectiveness of the inhalation devices in terms of delivering improved clinical outcomes.
A systematic review of the effectiveness of different inhaler devices in asthma and COPD noted problems in comparisons between studies because of variation in the relevant inhaler technique checklists.16 The objective of the present review was therefore to describe and compare the inhaler technique checklists and scoring systems reported for Turbuhaler and Diskus and to recommend uniform checklists and scoring systems for use in future studies and in clinical practice.
Review of the Literature
Search Strategy
The search strategy included PubMed, Embase, and International Pharmacy Abstracts up to 2012. Key words used for the search included “inhaler technique,” “checklists,” “Turbuhaler,” “Turbohaler,” “Accuhaler,” “Diskus,” and “incorrect use.” The titles and abstracts of all articles produced by this search were assessed for inclusion before retrieval of full articles. These articles were then subsequently reassessed for inclusion, and only those presenting checklists for the Turbuhaler and/or Diskus and meeting the inclusion criteria were included in the review. No blinding of authors' names or institutions was done, and no scoring system for study quality was used. The inclusion criteria were studies of Turbuhaler or Diskus usage involving subjects with asthma or COPD and/or health care professionals. Only publications in the English language were included. Letters to editors, commentaries, cost analyses, and surveys were excluded. Instructions packaged with the inhaler devices themselves were also included.
Published Inhaler Technique Checklists and Reporting Systems
Twenty-six research articles were included in the analysis of inhaler technique checklists for the Turbuhaler and Diskus. Table 1 lists these checklists, together with the list of steps in the manufacturers' instructions.
Checklists Used in the Assessment of Inhaler Technique in Clinical Studies for the Turbuhaler and Diskus
Number and Categorization of Steps
The 24 different checklists for the Turbuhaler and 16 for the Diskus. It shows substantial variation between checklists in the number of steps listed, ranging from 3 to 14 steps for the Turbuhaler and from 3 to 13 steps for the Diskus. Most checklists included removing the Turbuhaler cap (19/24) or opening the Diskus cover (14/16), and all checklists included steps for loading a dose and inhaling the dose. However, first exhaling away from the mouthpiece was listed in fewer than half of checklists for both devices. The speed of inhalation, most often described as needing to be strong and forceful, was not mentioned in 4 of 24 Turbuhaler checklists and in 3 of 16 Diskus checklists. Almost all checklists included breath-holding after inhalation (21 of 24 for the Turbuhaler and 13 of 16 for the Diskus). Some checklists also included an item for waiting a specified time between doses,22,24,25 and one included rinsing and spitting out after use of the inhaler.22
Some checklists (10 for the Turbuhaler and 6 for the Diskus) further identified a subset of steps as being essential or critical; these are indicated in italics in Table 1. The number of these essential steps varied, most commonly 4 for the Turbuhaler and 3 for the Diskus. For the Turbuhaler, the essential steps were usually holding the device upright during loading, twisting the base around and back to load a dose, exhaling away from the device, and inhaling strongly and forcefully. For the Diskus, the most common essential steps were opening the mouthpiece, pushing the lever to load a dose, and inhaling. However, the essential items varied substantially between checklists. In most cases, the authors stated that the selection of an item as essential or critical was based on their evaluation that if this step was incorrectly performed, little or no medication would reach the lungs.
Other authors classified some steps as common problem steps.36 van Beerendonk et al40 divided the checklist into skilled and non-skilled items, stating that non-skilled items, such as exhaling to residual volume, required only information, whereas skilled items, such as inhaling forcefully and deeply, also required physical training.
Scoring and Reporting Systems for Inhaler Technique Checklists
As with checklist items, scoring systems varied greatly between publications. The most common approach was to give a score of 1 for each correctly/satisfactorily performed step and a score of 0 for each skipped or incorrectly/unsatisfactorily performed step.14,20,22,24,36,41,44 Kesten et al25 graded pharmacists' Turbuhaler skills as good, fair, adequate, or skipped, with a score of 0 for skipped/not adequate and a score of 1 for good/fair. Rönmark et al38 allocated a score of 2 for correct, 1 for not completely correct, and 0 for incorrect.
Some checklists had a more complicated scoring system. Steier et al44 gave a total score of 0 if a step was left out that was necessary for successful inhalation (eg, opening of the cap, loading) or if the patient exhaled into the device, and all other attempts were rated from 1–9 points. Sestini et al42 gave a score of 1 for each item of the checklist considered as minor and a score of 3 for each one considered as major.
For reporting a patient's overall inhaler technique, most authors reported the inhaler technique score as the sum of the scores for each step, or as the proportion of checklist items that were performed correctly. More complicated systems were used by some authors. Rönmark et al38 assigned overall correct use of the device if no step was performed incorrectly (score of 0) and if no more than 2 of 4 specific steps (steps 2, 3, 5, and 7 in Table 1) were not completely correct (score of 1). Lenney et al29 provided 3 checklists for each device: the first (A, called Optimal Technique) included all steps required to use the device correctly, the second (B, called Some Delivery) included 2–3 common errors relevant to the device, and the third (C, called Little or No Delivery) included more substantial errors. Each patient's technique was graded as A, B, or C according to the checklist to which it best corresponded.
For summarizing population results, the most common approaches were to report the average per-patient score and/or the proportion of patients who demonstrated all checklist steps correctly or who had any errors.18,43,46,47 van der Palen et al18 and Basheti et al19,46,47 also reported the proportion of patients with correct essential technique (all essential steps in Table 1 correct), and Melani et al33 reported the proportion of patients with any critical errors. Some authors also reported data for each individual checklist step, reporting the proportion of patients performing each step correctly43 or incorrectly.33
Source of Information for Checklists
It was not always possible to determine the source of a published checklist (Table 1). Authors often stated that they had based their checklists on the manufacturers' leaflets,20,21,28,38,42 previously published checklists,23,31 or national and international asthma foundation guidelines18,22 and/or that they developed their own. Personal or expert opinion was often used to justify the checklist items; for example, Nimmo et al37 stated that the Turbuhaler checklist steps were those “identified as necessary for correct inhalation technique for the purpose of the study.”
Summary
The above body of literature demonstrates that there is no defined standard for assessment of inhaler technique with the Turbuhaler or Diskus with regard to the number and content of steps in the relevant checklists. Not all studies stated the source of their checklists, and even when the source of the checklists was stated, there were many different sources. Scoring systems used to present the results of the assessments were very different as well, making it unfeasible to compare the results in many cases. Therefore, although overall similar processes are described in each checklist, the range of content and format presents a challenge with regard to drawing comparisons between the checklists and validation of the individual steps.
A recent review has called for standardization of the way inhaler device studies are conducted and the way they are reported to physicians and health authorities.48 Considering the above findings, there are several obvious questions at this stage. Which checklists are most appropriate to be used for future studies assessing and/or educating patients on correct Turbuhaler and/or Diskus technique? Which steps should be weighed more, that is, which steps can be considered essential steps? How should inhaler technique data be scored and reported? To answer these questions, we examined evidence for the clinical importance of each of the steps commonly included in checklists for the Turbuhaler and Diskus.
Evidence for Inclusion of Individual Technique Steps in Device Checklists
Each checklist deals with 3 overall processes: preparation of the device/loading of the dose, delivery of the dose, and preparation of the device for storage. Within these 3 overall processes, we identified published evidence that supports individual checklist steps. It is not possible to find evidence for some of the steps that are included in the manufacturers' instructions for a particular device; these recommendations may have been developed in-house, with various options tested and some perhaps abandoned, and the evidence is not necessarily available in the public domain.
Preparation of the Device.
Most checklists describe the preparation of the device to include (1) removing the cover (Turbuhaler) or opening the cover (Diskus) and (2) loading a dose for delivery.
(1) Although there is no published evidence on the importance, necessity, or impact of removing the cover (Turbuhaler) or opening the cover (Diskus), the design of the devices means that it would be physically impossible for any medication to be delivered if this step was not performed. This may seem so obvious as to be unnecessary to check, but a small proportion of patients fail to complete this step.34
(2) Loading (or preparing) a dose for delivery is described for both the Turbuhaler and Diskus. For the Turbuhaler (see Fig. 1), the medication powder is stored in bulk in a reservoir (storage unit for drug), above a dose well (dosing unit), and prior to drug administration, a single dose of power needs to be aliquoted (loaded).49 This is achieved by holding the device upright and rotating the grip at the base of the Turbuhaler in one direction and back again. Although early Turbuhaler instructions specified starting with a counterclockwise rotation, the manufacturer later clarified that the direction of initial rotation was irrelevant provided the base was rotated in both directions, and a click was heard. If the device is upright, the drug falls by gravity from the reservoir into the dosing unit, and excess powder is scraped off to make the measured dose available.6,50,51 This is an important step because, without it, an appropriate dose of medication will not be available for delivery.
In contrast, the Diskus contains a coiled strip containing metered doses of powder (see Fig. 2). When the lever is pulled to the right, the 2 foil strips are peeled apart, and the dose is aligned with and exposed to the mouthpiece. Without the lever being pulled across, it is physically impossible for any medication to be inhaled.
Delivery of the Dose.
This process involves all of the breathing maneuvers required to move the drug from the device and into the body. Typically, the checklists involve a description of (1) how to exhale the air from the lungs (how much and where), (2) how to place the mouthpiece of the device into the mouth, (3) how to inhale the medication from the device, and (4) breath-holding after inhalation.
(1) Exhaling air prior to inhalation is a physiological process. Although some checklists do not include this step on the basis that it does not affect clinical outcomes,52 other checklists specify exhaling to residual volume, based on clinical experience that this ensures that the patient can make a deep and forceful inhalation.19,47 For both the Turbuhaler and Diskus, it is important to exhale away from the mouthpiece, as there is evidence that exhalation into the device may reduce the dose delivered to the lung.53–55 Exhalation into or near the device compromises the integrity of the powder dose: for both the Turbuhaler12,49,51,53,56,57 and the Diskus,58 it can displace the already loaded dose of powder and introduce moisture, potentially causing aggregation of powder particles.
(2) Placing the mouthpiece between teeth and lips before inhalation (Turbuhaler and Diskus) may also influence clinical outcomes.58 A tight seal between the lips and mouthpiece avoids air leakage, which would otherwise reduce the inspiratory flow. Placing the mouthpiece past the lips and teeth is important; otherwise, a portion of the aerosol particles may deposit onto the teeth and tongue, decreasing the amount of medication reaching the lungs.58
(3) Inhaling forcefully and deeply (ie, generating sufficient inspiratory flow) has been shown to be important for optimal drug delivery for the Turbuhaler and Diskus,6,29,59 as it ensures de-aggregation of the metered dose60 and deep lung deposition.58,61 Inspiration should be forceful from the beginning of inhalation rather than increasing gradually.57 Failure to do so results in partial delivery of the medication to the lungs29 and increased drug deposition in the mouth and pharynx.57,60,62,63 Maintaining a forceful and deep inhalation from the start of the inhalation maneuver and for as long as possible ensures that most patients can use a powder inhaler (Turbuhaler and Diskus) irrespective of the resistance of the device or their disease severity.64 However, although most patients can achieve the required flow,65,66 flow is more likely to be a problem for the Turbuhaler than for the lower resistance Diskus.67,68
(4) Breath-holding following inhalation (Turbuhaler and Diskus) is included in most of the published checklists and the Global Initiative for Asthma web site checklists for both the Turbuhaler and the Diskus (www.ginasthma.org/Turbohaler; www.ginasthma.org/Accuhaler-(Diskus), Accessed June 6, 2014). This is one of the most frequently discussed items in inhaler technique checklists.52 Although the manufacturer's instructions for the Turbuhaler do not include this step, many clinicians advise inclusion of a breath-hold for powder inhalers to avoid confusion with pressurized metered-dose inhalers, for which a breath-hold is necessary.69 For pressurized metered-dose inhalers, the original suggestion of a 10-s breath-hold was based on lung deposition studies,69 but the general recommendation for clinical practice is that the breath should be held only as long as is comfortable. A 5-s breath-hold is a realistic target for most patients.70
Preparation of the Device for Storage.
Replacing the cap after use of a Turbuhaler or closing the lid of the Diskus is the final step articulated in inhaler checklists. Although this step is post-drug delivery, it serves to keep the device clean, prevent foreign objects from entering the mouthpiece, and minimize absorption of moisture from the ambient environment.58,71 Therefore these steps are important in maintaining the drug integrity for subsequent doses. For the Turbuhaler, replacing the cap is included in the manufacturer's instructions but in fewer than half of published Turbuhaler checklists.
Evidence for Additional Steps
There does not appear to be any published evidence to support a 30-s delay between doses, and it is not included in manufacturers' instruction leaflets in countries such as Jordan, Canada, Australia, and the United States. This step would only be relevant to patients prescribed 2 or more inhalation doses on each occasion. Likewise, an instruction to rinse the mouth after taking a dose, although important for patients using inhaled corticosteroids to reduce the risk of oropharyngeal side effects, is not relevant to those using the Turbuhaler or Diskus for delivery of short- or long-acting β2 agonists.
Nevertheless, if the perspective of Appel13 is adopted, that is, the better the inhaler technique (the more correct steps performed) by the patient, the better the clinical response expected,14,47 these additional steps should be considered while delivering a complete Turbuhaler and Diskus technique education. Patients for whom a second dose or mouth rinsing is relevant may need to be asked about these steps, as they may not think to perform them when asked to demonstrate their technique.
Essential Steps for the Turbuhaler and Diskus
As mentioned previously, in some published checklists, a series of steps have been identified as essential. Considering the evidence above, it becomes clear that, without the completion of certain steps, it is unlikely that any medication will be delivered to the airways. It is these steps that could be considered essential. They include opening/removing the cover; loading the device,49 which, in the case of the Turbuhaler, should be completed while holding the device upright18,50; and inhaling through the mouthpiece forcefully and deeply.57,62 Failure to perform these steps will result in no medication being available because of the physical barrier of the cap or lid, the unavailability of the drug, or the drug failing to be transported out of the inhaler.
From the literature, the published checklists for the Turbuhaler and Diskus that best describe all of the steps recommended are those published by van der Palen et al18 and Basheti et al14 (Table 2).
Recommended Checklists for Assessment of Turbuhaler and Diskus Technique
Reporting Turbuhaler/Diskus Technique Data
To allow comparison of data from different studies, we recommend that inhaler technique data should be presented as (1) the proportion of patients with correct technique (all steps in the checklist correct); (2) the proportion of patients with correct essential technique (all essential steps identified in each checklist correct); (3) the mean ± SD of inhaler technique scores; and (4) for more detailed studies, the proportion of patients with correct technique for each individual step in the technique checklist.
If all of these metrics are reported (or at least recommendations 1–3), appropriate comparison of populations will be possible in different locations and over time. In addition, the effectiveness of interventions designed to improve inhaler technique and thus clinical outcomes could be established.
Clinical Impact of Using Inhaler Technique Checklists
In the literature and in clinical practice, there is broad acceptance that poor inhaler technique is a substantial contributor to poor asthma control and to increased health care costs.49 For pressurized metered-dose inhalers, Giraud et al72 showed a strong relationship between poor technique, assessed with a standardized checklist, and a composite asthma instability index. A subsequent study by the same authors15 showed that assessment and correction of inhaler technique using a standardized checklist led to a significant improvement in asthma control, particularly in patients whose inhaler technique was suboptimal at the start.15 Although there have been many studies evaluating powder inhaler technique, a review by Lavorini et al56 found that few studies had documented the relationship between incorrect technique and clinical outcomes; however, there is no reason to suppose that this would be any different than for pressurized metered-dose inhalers. The concept of essential steps is supported by the finding of Melani et al33 that patients with one or more critical errors on the Turbuhaler or Diskus checklist had significantly worse asthma control, impaired quality of life, activity limitation, and sleep disturbance. The strongest confirmation of the value of inhaler checklists comes from studies in which inhaler assessment and skills training were associated with significantly improved asthma outcomes.14 Again, there are few studies, but in young children, Goren et al73 showed that improved Turbuhaler technique with a rudimentary child-specific checklist was associated with greater bronchodilator effect. In the 6-month study by Basheti et al,14 a brief pharmacy intervention, with assessment and correction of inhaler technique and checklist-based inhaler labels, led to significant improvement in asthma outcomes compared with a control group. In that study, changes in asthma control measures over time seemed to relate more to the proportion of patients with all steps correct than to average inhaler technique scores.14,47
Considering the difference in steps between Turbuhaler and Diskus checklists, another important practical recommendation that this review can add is that, where possible, only one type of inhaler device should be used per patient. Previous studies have shown that use of different devices predisposes patients to inhaler misuse compared with using only one type of inhaler.12,74,75 In addition, substituting a different powder inhaler for that previously used by the patient can cause confusion for the patient.76 Skilled health care professionals have an essential role in evaluating patients' inhalation technique and ensuring they are using the inhalers properly.12,57,68 Avoiding multiple inhaler devices for a patient can also lead to fewer steps to teach/learn and hence less confusion in demonstrating/performing the individual steps per inhaler device for health care professionals and patients.
Conclusions
Inhaler technique checklists have been used in the majority of studies investigating the inhaler technique performance of patients and of health care professionals such as pharmacists. This literature review demonstrated considerable heterogeneity in inhaler technique checklists for the same powder devices in different studies, which makes direct comparison of results difficult. This may cause confusion for both patients and health care professionals and ultimately contribute to poor technique. The issue of inhaler technique checklist heterogeneity was first raised more than a decade ago,16 but more work clearly needs to be done to ensure standardization of checklists for clinical and research contexts.
Checklists are feasible for use in clinical research and clinical practice, with no equipment costs and with minimal time required. Unlike existing inhaler technique assessment devices, which are able to check only some components of inhaler use, use of a checklist ensures that every step required for delivery of medication is assessed. However, the differences between the various checklists and scoring systems in the literature make the direct comparison of results difficult and at times inappropriate. Hence, in this review, we have recommended checklists and scoring systems for the most commonly used powder inhalers, the Turbuhaler and Diskus, based on the literature and previous findings. If future studies in this area use common checklists and scoring systems, the problem of inhaler technique assessment heterogeneity between different studies will be resolved, allowing for more accurate comparisons between the results.
From the literature, the published checklists for the Turbuhaler and Diskus that best describe all of the steps recommended above are those published by van der Palen et al18 and Basheti et al.14 The validity of these checklists is supported by evidence for significant improvement in asthma outcomes when technique is assessed and improved.14 It is recommended that future studies use these checklists so that direct comparison between studies can assess the efficacy of specific interventions.
Footnotes
- Correspondence: Iman A Basheti PhD, Faculty of Pharmacy, Applied Science University, PO Box 166, Amman 11931, Jordan. E-mail: dr_iman{at}asu.edu.jo.
The authors have disclosed no conflicts of interest.
- Copyright © 2014 by Daedalus Enterprises
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.
- 27.
- 28.↵
- 29.↵
- 30.
- 31.↵
- 32.
- 33.↵
- 34.↵
- 35.
- 36.↵
- 37.↵
- 38.↵
- 39.
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵