Introduction
Acute hypoxemic respiratory failure (AHRF) is a life-threatening complication of COVID-19 pneumonia. Noninvasive ventilation (NIV) might be useful in its initial management.1 However, vigorous inspiratory effort during spontaneous breathing (SB) may worsen lung injury, generating excessive lung stress and strain.2 Tonelli et al3 showed how the change of respiratory effort during NIV, and not oxygenation, was the earliest and most accurate parameter to predict NIV failure. Prone position (PP) might act in synergy with NIV to reduce respiratory efforts, by a more homogeneous ventilation distribution and reduced regional lung stress.4 Recent evidence showed that PP was able to reduce the magnitude of inspiratory effort during assisted ventilation and SB.5,6 Moreover, awake PP has been increasingly used over the past 2 years,7,8 with a meta-trial reporting a reduction in intubation rate.8 However, to our knowledge, no studies have evaluated whether the application of NIV may act synergically with PP in reducing inspiratory efforts in awake patients with COVID-19. To address this, we aimed to describe the combined effect of PP and NIV on the magnitude of respiratory effort and oxygenation in a cohort of subjects with COVID-19 affected by AHRF.
Methods
In this single-center prospective observational study, we screened consecutive patients admitted to our ICU of Papa Giovanni XXIII Hospital, Bergamo, Italy, between March 29–April 24, 2022. Inclusion criteria were age ≥ 18 y and confirmed diagnosis of COVID-19 pneumonia with AHRF. AHRF was defined as persistent PaO2/FIO2 ≤ 200 mm Hg and tachypnea (breathing frequency 25 breaths/min) or subjective dyspnea despite the initial oxygen therapy with non–rebreathing mask or noninvasive respiratory support (ie, CPAP, high-flow nasal cannula, NIV). Exclusion criteria were need for immediate intubation, preexisting neuromuscular disease, altered mental status, and noninvasive respiratory support > 48 h ongoing at the time of enrollment. The ethics committee approved the study (registration number 945/2021), and subject informed consent was obtained.
As part of our clinical practice for patients with COVID-19 requiring NIV, a multifunctional nasogastric tube (NutriVent, Sidam, Mirandola, Italy) was inserted and connected to a dedicated monitoring system (OptiVent, Sidam) to monitor esophageal pressure (Pes). Correct esophageal catheter placement was confirmed by (1) visualization of a negative deflection of Pes synchronous with inspiratory effort, (2) presence of cardiac artifacts on Pes tracings, and (3) radiopaque markers at chest radiograph. Once NIV was started, validation of Pes measurements was obtained through dynamic occlusion test.
NIV via full face mask was started, and after 30 min in supine position (SP), subjects were helped to prone. PP duration varied depending on subject’s tolerance and clinical judgment. If the subject refused to prone or asked to resume SP before a 2-h period, PP was considered unfeasible. NIV parameters, set at physicians’ discretion, were left unchanged during all the study phases.
Respiratory parameters and Pes swings (ΔPes, maximal negative deflection of Pes from the onset of inspiratory effort) were collected at 4 time points: (1) at baseline, with all subjects placed on Venturi or non–rebreathing mask (SB); (2) after 30 min of NIV in SP (S1); (3) after 2 h of NIV in PP (PP); and (4) after 30 min from resupination in NIV (S2). Sedation was permitted to maintain Richmond Agitation-Sedation Scale = 0. Richmond Agitation-Sedation Scale, days free of respiratory support within 28 d (NIV or invasive mechanical ventilation), need of endotracheal intubation, and ICU length of stay were recorded.
Continuous variables were presented as median [interquartile range], categorical data as count and percentage. The comparison between variables at 4 time points was performed using Friedman test and Dunn post hoc analysis and by Cochrane Q test. Correlation between data was verified by Pearson test.
Results
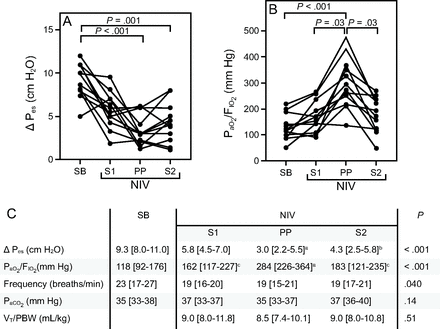
Among the 13 eligible patients one refused the nasogastric tube and was excluded. Twelve subjects were enrolled in the study and included in the analysis (83% male; age 60 [54–67] y; body mass index 27 [24–31] kg/m2). At the time of the study screening, 8 subjects were supported with CPAP (PEEP 7.5 cm H2O, FIO2 0.75 [0.71–0.79]) and 4 with non–rebreathing mask. The ongoing respiratory support was not increased and maximized, but we escalated directly to NIV+PP to limit the risk of further deterioration of the respiratory status. Duration from hospital admission to NIV start was 2 [1–4] d. All subjects completed the 2-h prone session. PP was maintained for a median of 240 [175–275] min. NIV parameters were left unchanged during the study: PEEP 8 [7–9] cm H2O, pressure support 8 [8–10] cm H2O, and FIO2 0.60 [0.60–0.60]. Compared to SB, ΔPes was decreased by the NIV application, although this reduction reached statistical significance only when PP was added (9.3 [8.0–11.0] cm H2O in SB vs 3.0 [2.2–5.5] cm H2O in PP; P < .001); this effect was maintained after resupination (4.3 [2.5–5.8] cm H2O in S2, P = .001) (Fig. 1A–C).
Esophageal pressure changes (ΔPes) and PaO2/FIO2 changes in individual subjects during the 4 phases of the study. A: Compared to spontaneous breathing (SB), ΔPes started declining with supine noninvasive ventilation (S1 vs SB, P = .09), and this reduction became significant in prone position (PP) (P < .001); this effect being maintained 30 min after resupination (P = .001, S2 vs SB). B: PaO2/FIO2 was significantly increased by PP compared to SB (P < .001), S1 (P = .03), and S2 (P = .03). After resupination, oxygenation did not significantly differ from baseline SB (P = .16 in SB vs S2). C: Ventilatory settings and gas exchanges over the 4 phases of the study. ΔPes = esophageal pressure changes; NIV = noninvasive ventilation; SB = spontaneous breathing with Venturi or non–rebreathing mask; S1 = after 30 min of supine NIV; PP = after 2 h of NIV in prone position S2 = after 30 min from resupination in NIV. VT = tidal volume; PBW = predicted body weight. P values were calculated by Friedman test for nonparametric repeated measure, and post hoc analysis was performed by Dunn test. (aP < .001 vs SB; bP = .001 vs SB; cP = .03 vs PP)
The PaO2/FIO2 was not improved by NIV alone, whereas it was significantly increased during PP (284 [226–364] mm Hg) compared to SB (118 [92–176] mm Hg, P < .001), S1 (162 [117–227] mm Hg, P = .03), and S2 (183 [121–235] mm Hg, P < .001) (Fig. 1B–C). This improvement was lost after resupination when PaO2/FIO2 did not differ from SB (183 [121–235] mm Hg in S2 vs 118 [92–176] mm Hg in SB, P = .16).
A moderate correlation between PaO2/FIO2 improvement and ΔPes reduction was observed (r = 0.5, r2 = 0.22, P = .002). No significant differences were found in PaCO2 and tidal volume/predicted body weight among the 4 time points; a reduction of breathing frequency was observed once NIV was started (Fig. 1C). Days free of respiratory support at 28 d were 18 [12–24] d; 3 (25%) subjects were intubated; ICU length of stay was 6 [5–19] d.
Discussion
Our study found that, in a small cohort of subjects affected by COVID-19 pneumonia, combining PP with NIV was associated with reduced inspiratory effort and improved oxygenation. Supine NIV decreased subjects’ respiratory effort, but was not statistically significant, potentially due to small sample size. When PP was applied, the reduction of respiratory effort became significant, suggesting a synergic effect of NIV and PP.
Yoshida et al5 recently described the impact of PP on reducing the magnitude of inspiratory effort in a cohort of subjects with ARDS under patient triggered ventilation. Similarly, we observed a cumulative effect of PP and NIV on reducing subjects’ inspiratory effort. This effect might be related to both a more homogeneous ventilation distribution and improved oxygenation.
Consistent with previous studies,9,10 oxygenation improvement was lost after resupination. Thus, PaO2/FIO2 improvement could not be explained only by a longer application of noninvasive support during PP compared to supine; but it might be related to a better ventilation/perfusion matching, which is a well-known effect of PP.
Limitations are the small number of subjects and the exploratory nature of our study, the inability to extrapolate data to non–COVID-19 causes of AHRF, and the short interval of prone positioning. To our knowledge, this is the first study assessing the combined impact of PP and noninvasive support on the magnitude of inspiratory effort. This approach needs further investigation.
Footnotes
- Correspondence: Lucia Zacchetti MD, Department of Anesthesia and Intensive Care Medicine, Papa Giovanni XXXIII Hospital, Piazza OMS, 1, 24127 Bergamo, Italy. E-mail: Lzacchetti{at}asst-pg23.it
The authors have disclosed no conflicts of interest.
Dr Punzi presented a version of this paper at ESICM 34th Annual Congress, held virtually October 3–6, 2021.
- Copyright © 2023 by Daedalus Enterprises