Introduction
Prone positioning is known to reduce mortality among patients on mechanical ventilation with moderate-to-severe ARDS.1 When extrapolating data about these patients with ARDS, awake prone positioning is touted as a low-risk intervention that can be performed outside the ICU. Studies show that awake prone positioning is feasible and effective in improving oxygenation in subjects with COVID-19 related pneumonia who are not intubated.2,3 In our recently published multi-center randomized controlled meta-trial, we found that awake prone positioning significantly reduced the composite outcome of intubation or death within 28 days of enrollment for subjects with COVID-19 and with acute hypoxemic respiratory failure supported by high-flow nasal cannula (HFNC) oxygen therapy.4 The early identification of patients at high risk of failure of awake prone positioning may help avoid delayed intubation, which might ultimately improve outcomes. However, predictors of awake prone positioning success remain largely unknown. Thus, we aimed to explore predictors of awake prone positioning success in subjects who were not intubated.
Methods
This was a post hoc analysis of the American data set from the meta-trial.4 Subjects assigned to the awake prone positioning arm and receiving awake prone positioning for ≥30 min were included. Additional data, including subject response to awake prone positioning on the second and third days of enrollment, had been prospectively collected in the American data set only were analyzed and reported. Treatment success was defined as a subject being discharged from the hospital alive without invasive ventilatory support after 28 days of enrollment. In this trial, the subjects were required to be in the prone position until intubation or death or until HFNC was discontinued based on predetermined criteria of 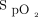 of 92–95% on HFNC flow at 40 L/min and
of 92–95% on HFNC flow at 40 L/min and 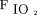 of 0.4. The original multi-center randomized controlled trial was registered in clinicaltrials.gov (NCT04325906) and approved by all the participating hospitals' ethics committees (approval 20032604-IRB01 in the leading hospital).
of 0.4. The original multi-center randomized controlled trial was registered in clinicaltrials.gov (NCT04325906) and approved by all the participating hospitals' ethics committees (approval 20032604-IRB01 in the leading hospital).
Continuous variables were reported as mean ± SD or median (interquartile range [IQR]) and compared by using the Student t test or the Mann-Whitney test. Categorical variables were reported as proportions and compared by using the chi-square test or the Fisher exact test. Binary stepwise logistic regression was carried out to determine the impact of age, cancer, 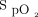 /
/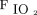 at study enrollment, ROX index (
at study enrollment, ROX index (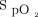 /
/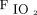 /breathing frequency) at study enrollment, mean daily duration of awake prone positioning, and
/breathing frequency) at study enrollment, mean daily duration of awake prone positioning, and 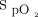 /
/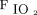 improvement on day 2 on the likelihood of treatment success. The accuracy of the variables to predict treatment success was assessed by calculating the area under the receiver operating receiver operating characteristic curves. Statistically significant independent variables were maintained in the model. A two-sided P < .05 was considered statistically significant. Data analysis was conducted by using SPSS 26.0 (SPSS, Chicago, Illinois).
improvement on day 2 on the likelihood of treatment success. The accuracy of the variables to predict treatment success was assessed by calculating the area under the receiver operating receiver operating characteristic curves. Statistically significant independent variables were maintained in the model. A two-sided P < .05 was considered statistically significant. Data analysis was conducted by using SPSS 26.0 (SPSS, Chicago, Illinois).
Results
From April 2, 2020, through January 26, 2021, 112 subjects were enrolled in the awake prone positioning group. Four subjects were excluded from this analysis due to being in the prone position for <30 min. One hundred eight subjects (69 men) were included, with a mean age of 62 y, of whom 65 (60%) had treatment success. Compared with the treatment failure group, the subjects in the treatment success group had a higher median (IQR) 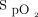 /
/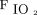 (156.7 [129.3–183] vs 134.3 [111.3–156.7]; P = .005) and median (IQR) ROX index (6.7 [5.1–8.1] vs 5.1 [4.0–7.5]; P = .02) at study enrollment, whereas no other significant differences in age, sex, body mass index, Sequential Organ Failure Assessment scores, and treatments were found between the groups (Table 1).
(156.7 [129.3–183] vs 134.3 [111.3–156.7]; P = .005) and median (IQR) ROX index (6.7 [5.1–8.1] vs 5.1 [4.0–7.5]; P = .02) at study enrollment, whereas no other significant differences in age, sex, body mass index, Sequential Organ Failure Assessment scores, and treatments were found between the groups (Table 1).
Subject Baseline Characteristics and Outcomes
In the first awake prone positioning session, 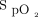 /
/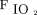 and ROX index were significantly improved in both treatment success and failure groups (Fig. 1), and the improvement of
and ROX index were significantly improved in both treatment success and failure groups (Fig. 1), and the improvement of 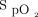 /
/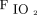 was similar in both groups (treatment success vs failure, 14.3 [2.9, 33.6] vs 7.5 [2.2, 40.8]; P = .48). However, only the subjects in the treatment success group had a significant improvement of
was similar in both groups (treatment success vs failure, 14.3 [2.9, 33.6] vs 7.5 [2.2, 40.8]; P = .48). However, only the subjects in the treatment success group had a significant improvement of 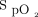 /
/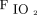 and ROX index with awake prone positioning on the second day and a significant improvement of
and ROX index with awake prone positioning on the second day and a significant improvement of 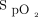 /
/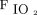 on the third day. The median (IQR)
on the third day. The median (IQR) 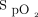 /
/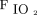 improvement to awake prone positioning on the second day was greater in the treatment success versus the treatment failure group (12.0 [3.3–31.0] vs 3.9 [0.5–12.0]; P = .003). Compared with the treatment failure group, the subjects in the treatment success group had a shorter median (IQR) HFNC duration (4.2 [2.1–7.2] d vs 6.9 [2.5–11.5] d; P = .037) and a shorter median (IQR) need-to-prone days (defined as the duration from study enrollment to HFNC discontinuation or intubation/death) (3.9 [1.9-7.1] vs 6.6 [2.5–11.9]; P = .009), but similar time spent on awake prone positioning at the daily average in the need-to-prone days (Table 1).
improvement to awake prone positioning on the second day was greater in the treatment success versus the treatment failure group (12.0 [3.3–31.0] vs 3.9 [0.5–12.0]; P = .003). Compared with the treatment failure group, the subjects in the treatment success group had a shorter median (IQR) HFNC duration (4.2 [2.1–7.2] d vs 6.9 [2.5–11.5] d; P = .037) and a shorter median (IQR) need-to-prone days (defined as the duration from study enrollment to HFNC discontinuation or intubation/death) (3.9 [1.9-7.1] vs 6.6 [2.5–11.9]; P = .009), but similar time spent on awake prone positioning at the daily average in the need-to-prone days (Table 1).
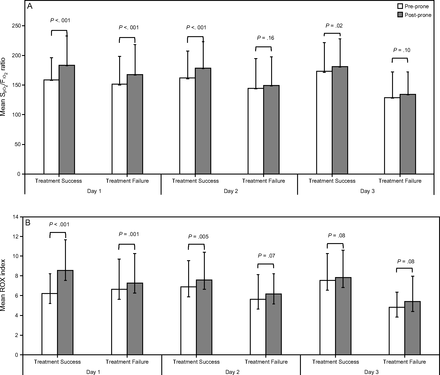
Oxygenation response to awake prone positioning in the first 3 days between treatment success and failure groups. In the first day, subjects’ 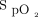 /
/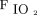 (A) and ROX index (B) significantly improved after awake prone positioning in both treatment success and failure groups. However, in the second day, only the subjects in the treatment success group had significant improvement of
(A) and ROX index (B) significantly improved after awake prone positioning in both treatment success and failure groups. However, in the second day, only the subjects in the treatment success group had significant improvement of 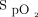 /
/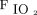 and ROX index after awake prone positioning. Similarly, on the third day,
and ROX index after awake prone positioning. Similarly, on the third day, 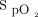 /
/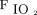 still significantly increased after awake prone positioning in the treatment success group but not in the treatment failure group. ROX=
still significantly increased after awake prone positioning in the treatment success group but not in the treatment failure group. ROX= 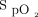 /
/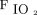 /breathing frequency.
/breathing frequency.
Logistic regression analysis identified that higher 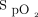 /
/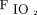 at study enrollment (odds ratio [OR] 1.022, 95% CI 1.007-1.037; P = .004) and
at study enrollment (odds ratio [OR] 1.022, 95% CI 1.007-1.037; P = .004) and 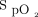 /
/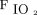 improvement to awake prone positioning in the second day (OR 1.051, 95% CI 1.016-1.087; P = .004) were associated with treatment success. The optimum cutoff value identified by drawing receiver operating characteristic curves for
improvement to awake prone positioning in the second day (OR 1.051, 95% CI 1.016-1.087; P = .004) were associated with treatment success. The optimum cutoff value identified by drawing receiver operating characteristic curves for 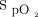 /
/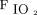 at study enrollment was 150, with a sensitivity of 0.646 and specificity of 0.674; whereas the cutoff value for
at study enrollment was 150, with a sensitivity of 0.646 and specificity of 0.674; whereas the cutoff value for 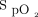 /
/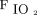 improvement to awake prone positioning in the second day was 11.5, with a sensitivity of 0.532 and specificity of 0.763. More subjects with
improvement to awake prone positioning in the second day was 11.5, with a sensitivity of 0.532 and specificity of 0.763. More subjects with 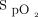 /
/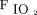 at study enrollment of ≥ 150 had treatment success than did subjects with
at study enrollment of ≥ 150 had treatment success than did subjects with 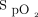 /
/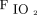 at study enrollment < 150 (75% vs 44%; P = .002). The subjects in the treatment success group had less use of noninvasive ventilation (8% vs 47%; P < .001), shorter median (IQR) ICU stay (5.0 [2.1–7.0] d vs 21.8 [17.2–35.5] d; P < .001), and shorter median (IQR) hospital stay (10.7 [8.5–14.9] d vs 24.5 [17.6–42.5] d; P < .001) versus the treatment failure group.
at study enrollment < 150 (75% vs 44%; P = .002). The subjects in the treatment success group had less use of noninvasive ventilation (8% vs 47%; P < .001), shorter median (IQR) ICU stay (5.0 [2.1–7.0] d vs 21.8 [17.2–35.5] d; P < .001), and shorter median (IQR) hospital stay (10.7 [8.5–14.9] d vs 24.5 [17.6–42.5] d; P < .001) versus the treatment failure group.
Discussion
In this study, we found that significant improvements in 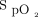 /
/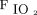 at day 2 and higher pre-prone
at day 2 and higher pre-prone 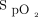 /
/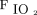 were possible predictors of awake prone positioning treatment success among subjects with COVID-19 induced acute hypoxemic respiratory failure. We found a higher
were possible predictors of awake prone positioning treatment success among subjects with COVID-19 induced acute hypoxemic respiratory failure. We found a higher 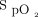 /
/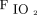 and ROX index before awake prone positioning in the treatment success group compared with the treatment failure group. This suggests a less-severe degree of respiratory failure in the treatment success group. Alternatively, this suggests that, within the inclusion group, the subjects with a higher
and ROX index before awake prone positioning in the treatment success group compared with the treatment failure group. This suggests a less-severe degree of respiratory failure in the treatment success group. Alternatively, this suggests that, within the inclusion group, the subjects with a higher 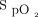 /
/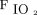 and ROX index benefitted the most from awake prone positioning because these subjects were potentially earlier in the spectrum of inflammation at the point of intervention. This is in accordance with analysis of our recently published data that suggests that early awake prone positioning had greater benefit than late awake prone positioning,5 which might imply a therapeutic window for subjects to benefit from awake prone positioning.
and ROX index benefitted the most from awake prone positioning because these subjects were potentially earlier in the spectrum of inflammation at the point of intervention. This is in accordance with analysis of our recently published data that suggests that early awake prone positioning had greater benefit than late awake prone positioning,5 which might imply a therapeutic window for subjects to benefit from awake prone positioning.
The prognostic value of subject responses to prone positioning during the first prone session for subjects with ARDS who were intubated was explored by van Meenen et al.6 When using changes in oxygenation, dead space, and driving pressure, they found no significant differences between survivors and nonsurvivors. In our previous study that evaluated subjects with ARDS induced by COVID-19 and who were intubated, we found significant improvements in oxygenation during the first prone session in the subjects who survived and in those who died or were placed on extracorporeal membrane oxygenation.7 However, survivors continued to respond to prone positioning during the second and third prone positioning sessions compared with no significant improvements among those who died or needed extracorporeal membrane oxygenation. The aforementioned results agree with the findings in our current study, which demonstrated that continued response to awake prone positioning on day 2 predicts treatment success. The treatment success group may still be in the exudative phase with diffuse alveolar damage, which could respond to awake prone positioning.8 This hypothesis needs to be validated in future studies.
Given the poorer outcome in the group with 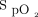 /
/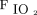 < 150 before awake prone positioning, close monitoring for response to awake prone positioning is needed for those subjects. Also, it is particularly important to assess their response to awake prone positioning on day 2, which might help decide the need for intubation. In addition, because a daily duration of awake prone positioning for at least 8 h/d was found to be associated with treatment success,4 efforts should be made to improve the tolerance of awake prone positioning among patients with pre-prone positioning
< 150 before awake prone positioning, close monitoring for response to awake prone positioning is needed for those subjects. Also, it is particularly important to assess their response to awake prone positioning on day 2, which might help decide the need for intubation. In addition, because a daily duration of awake prone positioning for at least 8 h/d was found to be associated with treatment success,4 efforts should be made to improve the tolerance of awake prone positioning among patients with pre-prone positioning 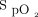 /
/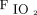 < 150, with the goal of avoiding intubation. The sample size of this post hoc analysis that examined the awake prone positioning subgroup might not be powered to comprehensively investigate predictors of awake prone positioning treatment success. This may explain why the ORs were marginally associated with treatment success for both
< 150, with the goal of avoiding intubation. The sample size of this post hoc analysis that examined the awake prone positioning subgroup might not be powered to comprehensively investigate predictors of awake prone positioning treatment success. This may explain why the ORs were marginally associated with treatment success for both 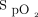 /
/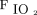 at enrollment and
at enrollment and 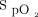 /
/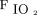 improvement to awake prone positioning on day 2. The smaller sample size might also explain the poor sensitivity and specificity for the cutoff values. Studies with a larger sample size are needed. In addition, we only enrolled subjects with COVID-19 and acute hypoxemic respiratory failure supported by HFNC. Thus, our findings cannot be applied to those with mild hypoxemia treated by invasive oxygen therapy or patients with acute hypercapnic respiratory failure. Our findings also cannot be generalized to patients without COVID-19.
improvement to awake prone positioning on day 2. The smaller sample size might also explain the poor sensitivity and specificity for the cutoff values. Studies with a larger sample size are needed. In addition, we only enrolled subjects with COVID-19 and acute hypoxemic respiratory failure supported by HFNC. Thus, our findings cannot be applied to those with mild hypoxemia treated by invasive oxygen therapy or patients with acute hypercapnic respiratory failure. Our findings also cannot be generalized to patients without COVID-19.
Pre-prone positioning oxygenation and oxygenation responses to awake prone positioning on the second day were associated with treatment success for subjects with COVID-19 and acute hypoxemic respiratory failure supported by HFNC. Further clinical trials are needed to confirm these findings. Closely monitoring patients with 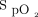 /
/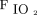 < 150 using HFNC before awake prone positioning, especially their response to awake prone positioning on the second day, may be helpful in identifying their need for intubation.
< 150 using HFNC before awake prone positioning, especially their response to awake prone positioning on the second day, may be helpful in identifying their need for intubation.
Acknowledgment
We thank Tyler Weiss MSc RRT RRT-ACCS; Lauren J. Harnois MSc RRT RRT-ACCS; Andrew Klein MSc RRT RRT-ACCS; Amanda Miller MSc RRT RRT-ACCS; Jacob Burd MSc RRT; Flor Cerda RN; Kathleen Posa-Kearney RN; Trevor W Oetting RRT; Lindsay Capouch RRT; Mark Greenwood RRT RRT-ACCS; and Scott Heckart RRT for their help on patient recruitment. We also thank the patients and relatives for their participation. We also thank clinical staff members of Respiratory Care Department, Rush University Medical Center, for providing their support to complete this study. We appreciate Rice foundation for sponsoring this study.
Footnotes
- Correspondence: Jie Li PhD RRT RRT-NPS RRT-ACCS FAARC, Division of Respiratory Care, Department of Cardiopulmonary Sciences, Rush University. 600 S Paulina St, Suite 765, Chicago, IL 60612. E-mail: Jie_Li{at}rush.edu
This study was supported by Rice Foundation. The funder had no role in study design, data analysis, the preparation or approval of the manuscript, or the decision to submit the manuscript for publication.
Dr Vines discloses relationships with Teleflex Medical, the Rice Foundation, and Theravance Biopharma. Dr Scott discloses relationships Teleflex and Aerogen. Dr Kaur discloses a relationship with the American Association of Respiratory Care. Dr Trump discloses a relationship with Fisher and Paykel. Ms Jackson discloses a relationship with Fisher & Paykel. Dr Li discloses relationships with Fisher & Paykel Healthcare, Heyer, Aerogen, the Rice Foundation, the American Association for Respiratory Care, and also serves as Section Editor for Respiratory Care. The other authors have no conflict of interest to disclose.
Drs Li, Mirza, and Vines conceived the idea. Drs Li, Kaur, Mirza, Scott, Mogri, Trump, and Morris, Mr Elshafei, and Ms Jackson implemented the study. Drs Li, Vines, Mirza, Trump, and Mogri supervised the study. Drs Mirza, Kaur, and Li conducted data analysis. Drs Mirza, Li, Vines, Kaur, Trump, and Mogri interpreted the data. Dr Mirza drafted the manuscript. Drs Li and Vines provided critical edits. All the authors reviewed the manuscript for important intellectual content and approved the final manuscript.
(ClinicalTrials.gov registration NCT04325906.)
- Copyright © 2022 by Daedalus Enterprises