Abstract
BACKGROUND: Exacerbations of COPD (ECOPD) are characterized by increased dyspnea due to dynamic pulmonary hyperinflation. This study sought to determine whether the AeroEclipse II breath-activated nebulizer (BAN) would produce greater bronchodilator responses than a continuous flow small-volume nebulizer (SVN) in patients with ECOPD.
METHODS: Prospective randomized controlled trial. Forty patients with ECOPD were recruited to participate in the trial. The primary study outcomes were inspiratory capacity (IC) and dyspnea via the Borg scale. Subjects were randomized to receive bronchodilator from either a BAN or a continuous flow SVN. Subjects in both groups received 2.5 mg albuterol sulfate and 0.5 mg ipratropium bromide by nebulizer every 4 hours, and 2.5 mg albuterol every 2 hours as needed. Approximately 2 hours after the subject's 6th scheduled nebulizer treatment, IC, dyspnea, and respiratory frequency measurements were repeated.
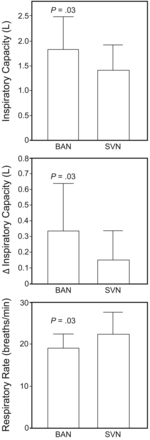
RESULTS: Both groups received an equal number of nebulizer treatments over the study period (BAN 6.25 ± 0.55, control 6.2 ± 0.7, P = .80). Following completion of the study protocol the BAN group had a higher IC than the SVN group (1.83 ± 0.65 L vs 1.42 ± 0.49 L, P = .03, respectively). The change in IC was higher in the BAN group (0.33 ± 0.31 L than in the SVN group (0.15 ± 0.19 L, P = .03). The BAN group also had a lower respiratory rate (19 ± 3.3 breaths/min vs 22 ± 5.3 breaths/min, P = .03, respectively). There was no difference in resting dyspnea as measured with the Borg scale (BAN 3.3 ± 2.1, SVN 3.5 ± 2.4, P = .69) or stay (BAN 4.6 ± 2.6 d, SVN 5.7 ± 2.8 d, P = .21).
CONCLUSIONS: In this cohort of patients with ECOPD, a BAN was more effective in reducing lung hyperinflation and respiratory frequency than a continuous-flow SVN.
Introduction
Exacerbations of COPD (ECOPD) are characterized by increased dyspnea, which is due in large part to dynamic pulmonary hyperinflation.1,2 The severe COPD exacerbation progressively increases both the central respiratory drive and oxygen cost of breathing while steadily consuming greater amounts of an often rapidly dwindling ventilatory reserve.3,4 If left unabated, respiratory muscle fatigue and ventilatory collapse may ensue, necessitating mechanical ventilation and a prolonged hospitalization.
Bronchodilator medications are commonly administered to patients with ECOPD with the intent of ameliorating dyspnea and dynamic hyperinflation.4 There is no compelling evidence that small-volume nebulizers (SVN) are superior to metered-dose inhalers in terms of bronchodilator response in patients with ECOPD.5 Nevertheless, SVN is very often selected as the aerosol delivery system in this clinical setting.
It is well known that the performance of SVN varies among commercially available models and that these differences impact the site and volume of aerosol deposition in the tracheobronchial tree.6,7 The AeroEclipse II breath-activated nebulizer (BAN, Monaghan Medical, Plattsburgh, New York) produces greater aerosol fine particle mass than a standard SVN.8 This study sought to determine whether the AeroEclipse II BAN would produce greater bronchodilator responses in patients admitted to the hospital with ECOPD.
QUICK LOOK
Current knowledge
Aerosolized bronchodilators are a mainstay of treatment for exacerbations of COPD. Bronchodilators are most frequently given by a continuous flow small-volume nebulizer.
What this paper contributes to our knowledge
The use of a breath-actuated nebulizer for bronchodilator delivery in exacerbations of COPD was associated with a reduction in lung hyperinflation and a decrease in respiratory rate, compared to a continuous flow small-volume nebulizer.
Methods
This study was conducted at St Joseph Hospital, Nashua, New Hampshire. The study protocol was approved by the institutional review board of St Joseph Hospital. All study subjects gave written informed consent prior to study enrollment.
Study Design
This was a prospective randomized controlled trial. This study was impossible to blind completely; however, efforts were made to blind clinicians as much as possible to minimize the influence of bias (these will be explained individually as they appear in the study protocol). Patients were studied with the intent-to-treat approach, in order to preserve the effect of group randomization.9
Subject Selection
Subjects were recruited via a convenience sample. Forty patients admitted to the hospital with a diagnosis of ECOPD were sought to participate in the trial. Patients admitted from the emergency department or directly from a physician office were eligible for enrollment. The primary study outcomes were inspiratory capacity (IC) and dyspnea via the Borg scale. In pre-test statistical analysis it was determined that 40 subjects would provide 80% statistical power to detect a ≥ 12% difference in Borg dyspnea scores and a ≥ 17% difference in IC between groups. Subjects were required to meet at least one of American Thoracic Society/European Respiratory Society (ATS/ERS) indications for COPD hospitalization.10 Subjects were recruited within 8 hours of hospital admission, in order to begin their study period from the very beginning of their hospitalization.
Several exclusion criteria were applied, including: age < 40 years (applied to avoid inclusion of asthmatic patients misclassified as having COPD); asthma; severe respiratory distress requiring immediate therapy; acute need for noninvasive ventilation; pneumonia accompanied by acute infiltrates on chest radiography; moderate to large pleural effusions; decompensated congestive heart failure; acute myocardial infarction; unstable angina; pulmonary embolism; lung cancer; diaphragm paralysis; sepsis; palliative care status; patients expected to die within 48 hours; confusion; delirium; dementia; medical necessity to use tiotropium instead of ipratropium; patients unable to perform required pulmonary function studies; FEV1 ≥ 70% of predicted and/or FEV1/FVC ≥ 70%; allergies or history of adverse drug reactions to albuterol or ipratropium; and inability to communicate verbally and fluently in the English language.
Study Protocol
Following written informed consent, subjects performed bedside spirometry testing (MedGraphics Ultima, Medical Graphics, St Paul, Minnesota). All spirometry testing was done in accordance with ATS/ERS recommendations.11 The National Health and Nutrition Examination Survey (NHANES) III reference values were applied to FEV1 measurements.12 IC was then measured using the same pulmonary function system, in accordance with ATS/ERS recommendations.13 Following pulmonary function testing, the following physiologic data were measured: dyspnea via a 0–10 Borg scale; respiratory frequency; heart rate; and functional oxygen saturation via pulse oximetry. A complete list of collected demographic data are listed in Table 1.
Subject Baseline Characteristics
After collection of baseline data, the investigator consulted with the computer generated randomization schedule for group assignment. Subjects were randomized to receive both nebulized albuterol sulfate and ipratropium bromide (Nephron Pharmaceuticals, Orlando, Florida) from either an AeroEclipse II BAN or a continuous flow SVN (AirLife Misty Max 10, CareFusion, Yorba Linda, California). Monaghan Medical provided the AeroEclipse II breath-actuated nebulizers used in this research study. The BAN was used in the breath-activated mode unless the patient was unable to trigger the device, in which case the continuous mode was used, with either a mouthpiece or a mask. In both groups the nebulizers were used to sputter. Randomization to treatment groups following collection of baseline data was purposeful, in order to blind the investigator to which group the subject would be assigned. In addition, the nebulizer used by each subject was stored in an opaque bag to blind the investigator collecting end of study data to which group the subject had been assigned.
Subjects in both groups received 2.5 mg albuterol sulfate and 0.5 mg ipratropium bromide (3 mL unit dose) by nebulizer every 4 hours, and 2.5 mg albuterol every 2 hours as needed. Common adverse effects associated with albuterol and ipratropium were monitored for and documented after every treatment. Approximately 2 hours after the subject's 6th scheduled nebulizer treatment (approximately 22 hours after enrollment) IC, dyspnea, and respiratory frequency measurements were repeated. These follow-up measurements ended the study period, and patients were treated from that point on according to physician and respiratory therapy protocol orders. Patients continued to use the nebulizer they were assigned from the study protocol. Whenever possible, the investigator collecting the end of study data was not the same investigator who performed the initial measurements. This arrangement was used whenever possible in order to blind the data collector to the subject's group assignment
Statistical Analysis
Group randomization and statistical computations were made using statistics software (Prism 4 and StatMate, GraphPad Software, San Diego, California). Data are expressed as mean ± standard deviation. Differences in continuous data were analyzed with an unpaired t test. Differences in categorical data were analyzed with the Fisher exact test. The Grubb test was applied to identify statistical outliers. A P value of < .05 was considered statistically significant.
Results
Forty-six patients were asked to participate in the study. Four patients declined entry after being read the informed consent document. Forty-two patients consented and were enrolled in the study. The enrollment of 2 subjects was terminated by the principal investigator: one subject could not perform pulmonary function testing correctly, and one subject had pulmonary function values that did not meet inclusion criteria. The remaining 40 subjects who were enrolled in the trial completed the study protocol. Ninety-seven percent of pulmonary function tests were performed by pulmonary function technologists. All pulmonary function measurements were examined by the primary investigator to ensure quality. The clinician collecting the end of study data was not the same clinician who performed the initial measurements in 78% of cases, with equal distribution between groups. There was no difference in baseline demographic data between the study and control groups (see Table 1).
Outcomes
Both groups received an equal number of nebulizer treatments over the study period (BAN 6.25 ± 0.55, control 6.2 ± 0.7, P = .80). Following completion of the study protocol the BAN group had a higher IC than the SVN group (1.83 ± 0.65 L vs 1.42 ± 0.49 L, P = .03, respectively). The change in IC was higher in the BAN group (0.33 ± 0.31 L than in the SVN group (0.15 ± 0.19 L, P = .03) (Figure). The BAN group also had a lower respiratory rate (19 ± 3.3 breaths/min vs 22 ± 5.3 breaths/min, P = .03, respectively). There was no difference in resting dyspnea as measured with the Borg scale (BAN 3.3 ± 2.1, SVN 3.5 ± 2.4, P = .69). There was no difference in stay between the groups (BAN 4.6 ± 2.6 d, SVN 5.7 ± 2.8 d, P = .21); however, this study was not designed or statistically powered to assess this outcome measure.
End of study absolute inspiratory capacity, Δ inspiratory capacity, and respiratory frequency in patients treated with a breath-activated nebulizer (BAN) and small-volume nebulizer (SVN).
Side Effects
There was no difference in adverse effects between groups (see Table 2). One subject in the BAN group had the albuterol dose reduced to 1.25 mg because of tremor. One subject in the SVN group had the albuterol dose reduced to 1.25 mg because of tremor and tachycardia. One subject in the SVN group had the nebulizer treatment changed to metered-dose inhaler (after 5 scheduled nebulizer treatments) because of dry mouth and sore throat. This subject remained in the study, in accordance with intent-to-treat analysis.9
Medication Side Effects
Discussion
There is no evidence that SVNs are superior to metered-dose inhalers in terms of bronchodilator response in patients with ECOPD5 or that treatment with both albuterol and ipratropium during ECOPD is superior to either agent alone14; however, this protocol is consistent with common clinical practice.
IC was chosen as one of the primary physiologic outcomes in this study because changes in FEV1 are often small over the course of an ECOPD,2 and monitoring of dynamic hyperinflation via IC and respiratory frequency may better reflect the response to therapy particularly in the early stages of an exacerbation.15 Stevenson et al2 studied pre and post-bronchodilator spirometry and lung volumes in patients hospitalized with ECOPD. At discharge the post-bronchodilator FEV1 had increased by only 90 mL while the post-bronchodilator IC rose by 230 mL. In addition, the improvement in FEV1 was found to be due to increases in volumes: not to improved forced expiratory flow (no change in FEV1/FVC ratio). Pinto-Plata et al15 studied pulmonary function, inflammatory cytokines, and other physiologic variables in patients hospitalized for ECOPD. Forty-eight hours after admission there was no change in FEV1 (0.87 L vs 0.94 L), while both dyspnea and IC had improved significantly. IC increased from 1.23 L to 1.46 L. The investigation found that ΔIC was more sensitive to physiologic improvements than ΔFEV1 and the ΔIC occurred sooner than ΔFEV1.
In this cohort of patients with ECOPD, the AeroEclipse II BAN was more effective than a continuous flow SVN in improving IC and reducing respiratory frequency. These improved physiologic outcomes observed in the BAN group did not translate into a more pronounced reduction in resting dyspnea. It may be that the observed differences in hyperinflation weren't large enough to affect resting dyspnea, but it is quite possible that dyspnea on exertion may have been affected by the observed improvement in IC in the BAN grouping. O'Donnell et al16 showed in a study of stable COPD patients that an increase of 0.3 L in IC following bronchodilator was large enough to increase exercise capacity. In the present study the mean increase in IC was 0.33 L in the BAN group and only 0.15 L in the SVN group. A fair criticism of this study's methodology is that an alternative method for assessing dyspnea, which asks subjects to reflect on their overall dyspnea instead of at one point in time, may have been more representative of the patient's response to therapy.17 Another limitation of this study protocol was that the response to therapy was evaluated only near the end of the first day of hospitalization. This study is therefore not able to report how physiologic outcomes may have responded to therapy over the course of the hospitalization. Stevenson et al2 documented important differences in Borg dyspnea scores on hospitalization days 2, 3, and day of discharge; however, similar changes were not observed for IC and respiratory rate. Pinto-Plata et al15 did observe statistically significant differences in respiratory rate and IC among patients hospitalized with ECOPD between baseline measurements and baseline plus 48 hours.
In this study there are 2 possible explanations for the superior physiologic outcomes observed in the subjects treated with the BAN. First, it may be that the BAN group simply received more medication because of the breath-activated mode (less environmental loss of aerosol). In an in vitro study, Rau et al18 showed that the AeroEclipse BAN in the breath-activated mode delivered 56% more aerosol than the Misty-Neb continuous flow SVN. In patients with ECOPD, lengthy expiratory times and frequent coughing can certainly be expected to reduce the delivered dose of bronchodilator from a continuous flow SVN. However, merely increasing the nominal dose of nebulized bronchodilator has not been shown to increase physiologic responses in patients with ECOPD. Nair et al19 studied the impact of doubling nebulized albuterol doses (2.5 mg vs 5 mg) in patients hospitalized with ECOPD. The investigators reported no difference in the recovery of FEV1 or peak expiratory flows between groups.
The second explanation for improved physiologic outcomes observed in the BAN group is superior nebulizer performance beyond the breath-activated feature. In patients with chronic air-flow obstruction, aerosol deposition in central airways is much greater than in peripheral airways. Ilowite and colleagues20 showed that the ratio of central to peripheral aerosol deposition was inversely related to FEV1. This phenomenon may limit the physiologic response to inhaled bronchodilator. Smaller aerosol particles are required to accomplish aerosol penetration deeper into the tracheobronchial tree.7 Zanen et al21 studied the optimal aerosol particle size for salbutamol and ipratropium in patients with severe COPD (mean FEV1 38% of predicted). Aerosols with a median mass aerodynamic diameter of approximately 3 μm produced the highest physiological responses in terms of FEV1 and specific airway conductance. Hess et al8 showed that the AeroEclipse nebulizer produced more fine particle mass (percent of particles < 4.7 μm multiplied by total aerosol output) than commercially available SVNs. Sangwan and colleagues22 conducted in vivo and in vitro comparisons of a standard SVN and the AeroEclipse nebulizer. The in vitro experiment showed that the AeroEclipse generated smaller particles than the SVN in both a standing cloud and during ventilation. Lung deposition imaging indicated that the AeroEclipse delivered more aerosol to the lungs than the SVN. Interestingly, the ratio of central to peripheral aerosol deposition was higher with the AeroEclipse nebulizer. It is impossible to know whether more peripheral aerosol penetration with the BAN had any role in the better physiologic responses recorded in this study of patients with ECOPD. However, targeting aerosol deposition to maximize therapeutic responses in patients with ECOPD would be an interesting focus of future studies.
Conclusions
In this cohort of patients with ECOPD, the AeroEclipse II BAN was more effective in reducing lung hyperinflation and respiratory frequency than a continuous-flow SVN. There was no difference in medication-related side effects between the BAN and the continuous-flow SVN. Confirmatory studies are indicated. In addition, future studies are needed to examine how a BAN might impact clinical outcomes such as stay, physiologic outcomes over the entire course of an ECOPD hospitalization, and the effectiveness of a BAN in the treatment of ECOPD managed in the out-patient setting.
Acknowledgments
Thanks to Lawrence Brundage MSc, Khalid Nahi RRT RPSGT, Gail Haynes RRT, Ronald Sargent CRT, Marla Landry CRT, Sandy Darosa RRT, and Andrew Murley CRT, St Joseph Hospital Respiratory Therapy Department. Without their assistance and clinical excellence this study could not have been conducted.
Footnotes
- Correspondence: Jeffrey M Haynes RRT RPFT, Department of Respiratory Therapy, St Joseph Hospital, 172 Kinsley Street, Nashua NH 03060. E-mail: jhaynes{at}sjhnh.org.
The author has disclosed a relationship with Monaghan Medical.
See the Related Editorial on Page 1524
- Copyright © 2012 by Daedalus Enterprises Inc.