Abstract
INTRODUCTION: Advanced air-flow limitation in patients with COPD leads to a reduction in vital capacity, respiratory muscle strength, and exercise capacity. However, its impact on chest and abdominal wall mobility is unknown. This study aimed to ascertain the prevalence of patients with COPD with reduced chest and abdominal wall mobility and to investigate the effect of reduced chest and abdominal wall mobility on pulmonary function, respiratory muscle strength, and exercise capacity.
METHODS: In 51 elderly male subjects with COPD, chest and abdominal wall mobility, FVC, FEV1, FEV1/FVC, maximal inspiratory pressure (PImax), maximal expiratory pressure (PEmax), and the 6-min walk distance (6MWD) were assessed. Chest and abdominal wall mobility were measured using the breathing movement scale (0–8) at the 3 regions (upper chest, lower chest, and abdomen). Reduced mobility was defined as a value lower than the lower limit of the normal scale. The unpaired t test, Mann-Whitney test, and multiple regression analysis were performed.
RESULTS: The percentages of subjects with reduced mobility were 78% for the upper chest, 76% for the lower chest, and 53% for the abdomen. The subjects with reduced mobility had significantly low FVC, FEV1, and 6MWD in each region and significantly low FEV1/FVC, PImax, and PEmax in the abdominal region compared with those with nonreduced mobility. FVC and 6MWD were independently associated with the scale values in each region and with the abdominal scale value, respectively.
CONCLUSIONS: The majority of subjects with COPD had reduced chest and abdominal wall mobility, which was independently associated with FVC. Even though abdominal wall mobility was relatively preserved compared with chest wall mobility, it was also independently associated with 6MWD.
Introduction
COPD is characterized by persistent air-flow limitation that is progressive. In most patients with COPD, the reduced elastic recoil of the lungs with expiratory flow limitation leads to hyperinflation of the lungs beyond the normal resting functional residual capacity.1 In the advanced stages of COPD, increased total lung capacity, functional residual capacity, and residual volume (RV) are observed, reflecting lung hyperinflation. Because of the increasing RV, vital capacity (VC) and inspiratory capacity decrease with the progression of air-flow obstruction.2,3
Dynamic hyperinflation reduces the pressure-generating capacity of the inspiratory muscles and limits maximum tidal volume, leading to exercise intolerance in patients with COPD.4,5 However, the cause of exercise limitation is complex and multifactorial.6 A few studies have reported that exercise limitation and dyspnea intensity during exercise are not necessarily associated with dynamic hyperinflation.7,8 Aliverti et al,7 using optoelectronic plethysmography, reported that subjects without hyperinflation had lower exercise tolerance than those with hyperinflation, but end-inspiratory chest wall and abdominal volumes remained constant during exercise. Therefore, it is possible that lack of chest and abdominal wall mobility could be a limiting factor in increasing end-inspiratory chest and abdominal volumes during exercise, causing exercise intolerance in patients with COPD.
Previous studies have reported a considerable decrease in chest wall mobility with aging and chronic chest diseases.9–11 Although associations between chest wall mobility, lung function, respiratory muscle strength, and exercise tolerance have been reported in patients with ankylosing spondylitis9,12,13 and fibromyalgia,14 there is a paucity of studies on chest wall mobility in patients with COPD. Malaguti et al15 reported that chest wall mobility is not associated with pulmonary function, although mobility at the abdominal level is associated with inspiratory capacity. However, whether chest and abdominal mobility are related to respiratory muscle strength and exercise tolerance remains to be investigated.
Chest wall mobility was assessed by measuring the chest wall circumference using a tape measure, which is a simple and inexpensive method in clinical practice. However, this method is inappropriate for assessing reduced mobility, because of the absence of defined reference values. Therefore, the assessment of the impact of reduced chest wall mobility on respiratory function with this method is limited.
To objectively and easily assess breathing movements, we developed a breathing movement scale, which rates chest and abdominal wall movements during quiet breathing and deep breathing on a scale of −1 to 8 (−1 to −3 for quiet breathing and 0–8 for deep breathing). Our previous studies demonstrated that breathing movement scale measurements using a simple pen-sized device (breathing movement-measuring device) had acceptable validity and reliability.16–18 In the breathing movement scale, reduced chest and abdominal wall mobility were defined as values below the lower limits of deep breathing movements of healthy people (scale value < 4). Therefore, using a breathing movement-measuring device for breathing movement scale measurements is a feasible method for revealing the features of reduced chest and abdominal wall mobility in patients with COPD.
This study aimed to ascertain the prevalence of patients with COPD who have reduced chest and abdominal wall mobility (assessed by breathing movement scale) and to investigate the effect of reduced chest and abdominal mobility on pulmonary function, respiratory muscle strength, and exercise tolerance.
QUICK LOOK
Current knowledge
Advanced air-flow limitation in patients with COPD leads to reduced lung volume, respiratory muscle strength, and exercise capacity. Chest and abdominal wall mobility is affected by these factors.
What this paper contributes to our knowledge
The majority of elderly male subjects with COPD, assessed by the breathing movement scale, had reduced chest and abdominal wall mobility, which was associated with FVC. Abdominal wall mobility was also independently associated with 6-min walk distance.
Methods
Participants
This study was performed at Takagi Hospital (Fukuoka, Japan) and Choseido-Watanabe Clinic (Saga, Japan). Fifty-one male subjects with COPD, who were >65 y old, participated in the study. They had been diagnosed according to the guidelines of the Global Initiative for Chronic Lung Obstructive Disease (GOLD) and had received out-patient pulmonary rehabilitation. The participants, who were in GOLD stages I to IV, were clinically stable and experienced no exacerbations or changes in medication for ≥ a month. Participants with heart diseases, asthma, bronchiectasis, pulmonary fibrosis, obvious scoliosis and kyphosis, or cognitive disorders that prevented comprehension of measurement instructions were excluded.
This study was approved by the ethics committee of the International University of Health and Welfare and Takagi Hospital, and all participants gave their written informed consent.
Chest and Abdominal Wall Mobility Measurements
The breathing movement scale for deep breathing, which is based on a scale of 0–8, was used to assess chest and abdominal wall mobility (Table 1). Breathing movements that ranged from the end of maximal expiration to maximal inspiration with the subject in the supine position were measured at 5 observation points for 3 regions: the right and left sides of the third rib (upper chest) and eighth rib (lower chest), along the vertical line through the medial one third of the clavicle, and the midpoint between the xiphoid process and umbilicus (abdomen) along the vertical line through the umbilicus. For the upper and lower chest regions, scale values were defined as the averages for the right and left sides. A scale value of the breathing movement scale <4 at each observation point was considered an indication of reduced mobility. The criterion for each scale value in the breathing movement scale was determined from the data of quiet breathing and deep breathing movements measured by 3-dimensional motion analysis in healthy people 20–74 y of age.19
Breathing Movement Scale
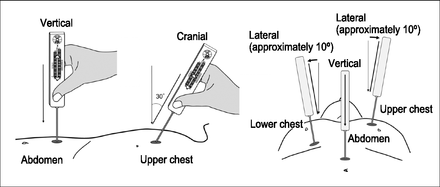
Scale values were measured using a custom-made breathing movement-measuring device (Pacific Medico, Tokyo, Japan) and were judged from the position of the mark on the breathing movement scale labels corresponding to the observation points (Fig. 1). Subjects were requested to remove their heavy outer clothing, such as sweaters and coats, and to loosen their pants while in the supine position, and then were instructed not to talk or move during the measurements. While assessing the breathing movements for upper and lower chest, the breathing movement-measuring device was held by hand at a 30° angle cranially and a slightly (approximately 10°) laterally inclined position from the vertical line (Fig. 2). For the abdomen, the device was kept in the vertical position. After stabilizing the elbow of the hand that held the device and setting the breathing movement-measuring device mark for reading the scale at the start point, the other breathing movement-measuring device mark was adjusted to the start point of the scale at the end of maximal expiration by pressing the device lightly against the observation point. Deep breathing from maximal expiration to maximal inspiration was performed at least twice, and the maximal scale value was recorded.
The breathing movement-measuring device is a pen-sized mechanical device. Breathing movement scale (BMS) values are measured from the position of the mark on the breathing movement scale labels with the device. The mark is moved along the label via the contact part while maintaining the device position at the measurement angle.
The breathing movement-measuring device was held by hand at a 30° angle cranially, at a slightly (approximately 10°) laterally inclined position from the vertical line for the upper and lower chest, and vertically for the abdomen while the subject took a deep breath. The scale values of each observation point were measured from the position of the mark on the breathing movement scale label with the device.
Spirometry
Spirometry was performed to determine FVC, FEV1, and FEV1/FVC with a spirometer (AS-507, Minato Medical Science, Tokyo, Japan), according to the American Thoracic Society and European Respiratory Society guidelines.20 FVC and FEV1 were measured 3 times and the largest value was recorded.
Respiratory Muscle Strength
Respiratory muscle strength was evaluated by measuring the maximal inspiratory pressure (PImax) and the maximal expiratory pressure (PEmax) using a pressure manometer (AS-507, Minato Medical Science) with the least air leakage. PImax and PEmax were measured with the subject in the sitting position, according to the recommendations proposed by the American Thoracic Society and European Respiratory Society.21 PImax was measured starting from RV, and PEmax was measured starting from total lung capacity. Tests were repeated at least 3 times, and the best value was recorded.
6-min Walk Test
The 6-min walk test (6MWT) was performed over a 10-m straight course as per the American Thoracic Society guidelines.22 Participants were repeatedly encouraged during the test using standardized phrases and were allowed to stop and rest during the test but were instructed to resume walking as soon as they felt able to do so. Pulse rate and transcutaneous oxygen saturation were assessed at rest and at the end of each minute during exercise using a portable pulse oximeter. Dyspnea was rated at rest and at the end of the test using a modified Borg scale (range 0–10). Two tests were performed, and the best 6MWD was recorded.
Statistical Analysis
The subjects were divided into 2 groups (nonreduced and reduced mobility groups) based on whether they had reduced mobility in each region. Data are summarized as mean ± SD for continuous variables and median, minimum, and maximum values for ordinal variables (scale values). The unpaired t test and Mann-Whitney U test were performed to compare the 2 groups for continuous variables with normal distribution and for continuous variables without normal distribution and ordinal variables, respectively. Normal distributions of continuous variables were verified with a Kolmogorov-Smirnov test. Stepwise multiple linear regression analyses were performed to identify any association between the scale values in each region with FVC, FEV1, PImax, PEmax, and 6MWD. Residual analyses were conducted to confirm normality, linearity, and equal variance of the regression model. A P <.05 was considered significant. Statistical analyses were performed using IBM SPSS Statistics 19 (IBM, Armonk, New York).
Results
The percentage of subjects with reduced mobility was 78% for the upper chest, 76% for lower chest, and 53% for abdomen (Table 2). Almost all of the subjects with GOLD stage III–IV COPD had reduced chest wall mobility, although the majority of the subjects with GOLD stage I–II COPD also had reduced chest wall mobility. The percentage of subjects with reduced mobility in the abdominal region increased progressively according to the GOLD stage. The characteristics of the reduced and nonreduced mobility groups for each region are shown in Table 3. There were no significant differences in age, weight, height, and BMI between the reduced mobility and nonreduced groups for each region. Scale values were significantly lower in the reduced mobility group than in the nonreduced mobility group for each region.
Prevalence of Reduced Chest and Abdominal Mobility by Global Initiative for Chronic Lung Obstructive Disease (GOLD) Stage
Comparisons of Characteristics and Clinical Variables Between the Nonreduced and the Reduced Mobility Groups in the Upper Chest, Lower Chest, and Abdominal Regions
Other variables analyzed for each region, including FVC, percent-of-predicted FVC, FEV1, percent-of-predicted FEV1, 6MWD, and each scale value were all significantly lower in the reduced mobility group than in the nonreduced mobility group (Table 3). For the abdominal region, significantly lower FEV1/FVC, PImax, and PEmax values were found in the reduced mobility group compared with the nonreduced mobility group.
In the multiple linear regression analysis, FVC was independently associated with scale values of the upper and the lower chest. FVC and 6MWD were independently associated with scale values of the abdomen (Table 4).
Results of the Stepwise Multiple Linear Regression Analyses for Determining the Factors of the Scale Values in the Upper Chest, Lower Chest, and Abdominal Regions
Discussion
The main findings of this study are that approximately three fourths of the elderly male subjects with COPD had reduced chest wall mobility, which was commonly associated with FVC, whereas abdominal wall mobility was relatively preserved compared with chest wall mobility and was also associated with 6MWD. To the best of our knowledge, our study is the first to demonstrate the prevalence of reduced chest and abdominal wall mobility in subjects with COPD, as assessed by the breathing movement scale, and also the first to associate this reduced mobility with pulmonary function, respiratory muscle strength, and exercise tolerance in subjects with COPD.
Previous studies showed that chest expansion declined with aging and chronic chest disease.9–11 Our study is in agreement with these previous studies, and our results demonstrate a high prevalence of reduced mobility in the upper and lower chest regions of the elderly male subjects with COPD, in particular, those with GOLD stage III–IV COPD. In addition, we found that in the upper and lower chest regions the reduced mobility group had lower lung function (FVC and FEV1) and exercise tolerance than the nonreduced mobility group. As a consequence, FVC was the independent factor of the upper and lower scale values. These results are consistent with previous studies reporting that chest wall mobility was closely associated with FVC in subjects with ankylosing spondylitis.12,13 Therefore, maintaining chest wall mobility may be an important element for preserving FVC in elderly male patients with COPD.
No significant differences in PImax were found between reduced and nonreduced mobility groups in the upper and lower chest regions. The external intercostal muscle is well developed in the upper ribs that have an inspiratory mechanical advantage.23 A previous study indicated that chest wall mobility was related to PImax and FVC in healthy subjects.24 Therefore, we assumed that there might be a significant difference in PImax between the reduced and nonreduced mobility groups in the chest regions. Even though the inspiratory intercostal muscles are shortened by pulmonary hyperinflation, it has been shown that the force-generating ability of these muscles remains almost unchanged.23 This may be one of the reasons for our findings in which there were no significant differences in PImax. Therefore, this result may imply that PImax is unlikely to contribute to chest wall mobility in elderly male patients with COPD.
The results of the comparisons between the reduced and nonreduced mobility groups differed in the abdominal region from those in the chest regions. In the abdominal region, the number of subjects in the reduced mobility group was comparable with that in the nonreduced mobility group. Abdominal wall mobility was relatively more maintained than the chest wall mobility. In subjects with limited ribcage expansion, such as the elderly25 and those with ankylosing spondylitis,26,27 it has been shown that limited chest wall expansion is compensated by increased abdominal wall movement to meet the ventilatory demand. The fact that abdominal wall mobility was relatively preserved may be attributed to the fact that approximately three fourths of the subjects with COPD had reduced chest wall mobility.
In addition, in the abdominal region, PImax and PEmax values in the reduced mobility group were significantly lower than in the nonreduced mobility group. Terzano et al28 reported that PImax and PEmax values were lower in subjects with severe airway obstruction than in subjects with mild and moderate airway obstruction. In the present study, the reduced mobility group in the abdominal region had significantly lower FEV1 values than the nonreduced mobility group had. Progressive decline in FEV1, largely reflecting a decrease in VC as a consequence of increased RV, has been suggested.3 Therefore, one explanation for significantly low PImax in the reduced mobility group in the abdominal region may be the reduced ability of the diaphragm to generate pressure due to an increase in RV (pulmonary hyperinflation).
On the other hand, it has been shown that physical activity was related to PEmax and 6MWD in subjects with COPD29 and PEmax in the elderly.30 Although physical activity was not assessed in this study, it has been reported that 6MWD was more closely associated with physical activity than with airway obstruction.31,32 In addition, Hopkinson et al33 indicated that fatigue of the abdominal muscle could be relevant to exercise tolerance. Considering that the abdominal muscles are recruited even during quiet breathing in patients with advanced COPD,34 it is likely that lower PEmax may be relevant to abdominal muscle fatigue following exercise. Therefore, we assume that the significantly low PEmax values in the reduced mobility group in the abdominal region may be associated with low 6MWD values.
In the stepwise multiple linear regression analysis, not only FVC but also 6MWD was a significantly independent variable for the abdominal scale. Alves et al35 showed that an increase in ventilation during exercise was associated with major motion of the abdominal compartment in subjects with COPD. Paulin et al36 observed that subjects with COPD with reduced diaphragmatic mobility had lower exercise tolerance although diaphragmatic mobility was more closely associated with air trapping than with exercise tolerance. Our recent study demonstrated that diaphragmatic mobility was closely associated with abdominal wall mobility (breathing movement-measuring device distance) in healthy, young males (r2 = 0.83) (unpublished data). These studies support our results that the abdominal scale is independently associated with 6MWD. Thus, the ability to change the volume of the abdominal compartment would play one of many important roles in ventilation during exercise in patients with COPD. Therefore, assessing chest and abdominal wall mobility by using the breathing movement scale would be helpful in exploring the impact of reduced chest and abdominal wall mobility on pulmonary function and exercise tolerance in elderly male patients with COPD.
However, the present study has some limitations. First, the sample size of the study was small. However, considering the sample size (α = .05, 1-β = .80) estimated from the effect size (f2 > 0.35) observed in the multiple linear regression analyses, it may be appropriate for the analyses. Second, the participants selected were only elderly male subjects with COPD to avoid the effect of sex and age on the observed measurements. Therefore, the results in our study cannot be generalized to elderly female or younger male and female patients with COPD. Third, reduced chest and abdominal wall mobility were judged by the lower limits of the normal scale value that was based on the data of healthy people 20–74 y of age. Because the mean age of the participants was 76 y, it is possible that the percentage of elderly male subjects with COPD having reduced mobility may have been overestimated. In our recent study, 31 healthy elderly males (77 ± 6 y), who had the predicted FVC > 80%, FEV1/FVC > 70%, and PImax > 40 cm H2O showed reduced mobility of 26% for the upper chest, 41% for the lower chest, and 3% for the abdomen (unpublished data). Fourth, chest and abdominal mobility was assessed with subjects in the supine position. Given that chest wall asynchronies are influenced by body position in patients with COPD,37 chest and abdominal mobility assessed by the breathing movement scale may not be totally reflected in chest and abdominal wall behavior in the upright position. Fifth, 6MWT was performed on a 10-m course. Beekman et al38 indicated that 6MWD on a 10-m course was shorter than on a 30-m course in subjects with COPD. Therefore, we assume that our results of 6MWD may be underestimated. However, because 6MWT of all the participants was conducted over the same course length, it should not have any influence on the results observed in this study. Finally, the subjects in this study had undergone out-patient pulmonary rehabilitation. Pellegrino et al39 reported that strenuous physical training for 2 months modified changes in lung volume during exercise. However, we believe that lung volume would not change in our subjects who had undergone mild to moderate exercise training.
Conclusions
A majority of the subjects with COPD had reduced chest and abdominal wall mobility, which was independently associated with FVC. Even though abdominal wall mobility was relatively preserved compared with chest wall mobility, it was also independently associated with 6MWD. Assessing chest and abdominal wall mobility using the breathing movement scale is helpful in exploring the impact of reduced mobility on pulmonary function and exercise tolerance in elderly male patients with COPD. Future studies will be needed to investigate whether an exercise program to improve chest and abdominal wall mobility would be helpful in improving exercise capacity.
Acknowledgments
We thank staff member Shusaku Koga for his support and measurements in this study.
Footnotes
- Correspondence: Hideo Kaneko PT PhD, Department of Physical Therapy, School of Health Sciences at Fukuoka, International University of Health and Welfare, 137-1 Enokizu, Okawa-shi, Fukuoka 831-8501, Japan. E-mail: hkaneko{at}iuhw.ac.jp.
Dr Kaneko presented a version of this paper at the 17th International Congress of the World Confederation for Physical Therapy, held May 1-4, 2015, in Singapore.
The authors have disclosed no conflicts of interest.
- Copyright © 2016 by Daedalus Enterprises