Abstract
BACKGROUND: Although the ratio of FEV1 to the vital capacity (VC) is universally accepted as the cornerstone of pulmonary function test (PFT) interpretation, FVC remains in common use. We sought to determine what the differences in PFT interpretation were when the largest measured vital capacity (VCmax) was used instead of the FVC.
METHODS: We included 12,238 consecutive PFTs obtained for routine clinical care. We interpreted all PFTs first using FVC in the interpretation algorithm and then again using the VCmax, obtained either before or after administration of inhaled bronchodilator.
RESULTS: Six percent of PFTs had an interpretive change when VCmax was used instead of FVC. The most common changes were: new diagnosis of obstruction and exclusion of restriction (previously suggested by low FVC without total lung capacity measured by body plethysmography). A nonspecific pattern occurred in 3% of all PFT interpretations with FVC. One fifth of these 3% produced a new diagnosis of obstruction with VCmax. The largest factors predicting a change in PFT interpretation with VCmax were a positive bronchodilator response and the administration of a bronchodilator. Larger FVCs decreased the odds of PFT interpretation change. Surprisingly, the increased numbers of PFT tests did not increase odds of PFT interpretation change.
CONCLUSIONS: Six percent of PFTs have a different interpretation when VCmax is used instead of FVC. Evaluating borderline or ambiguous PFTs using the VCmax may be informative in diagnosing obstruction and excluding restriction.
- respiratory function tests
- vital capacity
- airway obstruction
- spirometry
- respiratory physiological phenomena
- FEV1
- PFT
Introduction
Although the ratio of FEV1 to the vital capacity (VC) is universally accepted as the cornerstone of pulmonary function test (PFT) interpretation, disagreement and ambiguity remain regarding which VC to use. The 2005 American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines1 recommend using the largest VC. However, they do not specify whether or not the post-bronchodilator VC may be used. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines recommend using the FVC to calculate the FEV1/FVC and determine whether obstruction is present.2 Specifically, GOLD guidelines recommend using the post-bronchodilator FEV1/FVC to diagnose obstruction.2
It has long been recognized that obstructive lung physiology may exist even in cases of normal FEV1/(F)VC.1,3 Recommended strategies for diagnosing obstruction when FEV1 and FVC are reduced, but the total lung capacity (TLC) is normal, subsequently dubbed the nonspecific pattern, include repeat testing, measurement of slow VC (inspiratory or expiratory) for the FEV1/VC ratio, or bronchodilator testing.1 Indeed, the ATS/ERS guidelines note that in those cases, “significant improvement in FEV1, FVC, or both would suggest the presence of reversible air flow obstruction.”1
Although by the 2005 ATS/ERS guidelines,1 the nonspecific pattern was categorized as “obstruction,” clinically, it correlates predominantly with airway hyper-responsiveness and obesity.4 True to its name, however, it has also been reported in emphysema, bronchiectasis, pulmonary hypertension, diffuse parenchymal lung disease, sarcoidosis, and lung transplant.5 The nonspecific pattern has been shown to be stable in a large portion of patients over time, though a minority of patients transition to a restrictive or obstructive pattern.5,6 Predictors of which patients with nonspecific pattern on spirometry are likely to have an underlying obstructive or restrictive process are lacking.
Diagnosis of the nonspecific pattern on PFTs requires measurement of TLC by body plethysmography to exclude restriction. Lung volume testing is more expensive, more time-consuming, and less available than spirometry. However, reduced FVC with a normal FEV1/FVC has a low predictive value for restriction.7 Alternatively, a normal FVC on spirometry reasonably excludes restriction and the need for further lung volume testing.7 Thus, a diagnostic strategy to exclude restriction by spirometry in cases of normal FEV1/FVC and reduced FEV1 and FVC may help reduce unnecessary lung volume testing. Additionally, an interpretive strategy on spirometry may help to identify cases where the nonspecific pattern reflects airway hyper-responsiveness rather than a more restrictive process. In an era of increasing obesity, we may expect that the rate of nonspecific pattern diagnoses over time may continue to challenge clinicians.
Given the diagnostic uncertainty in cases of nonspecific pattern, as well as infrequent lung volume measurement, we sought to determine whether a PFT interpretive strategy that used the largest measured VC from any PFT test in a given session (whether that was the slow VC or the post-bronchodilator FVC) would help us to reduce the rate of nonspecific pattern diagnoses and reduce the need for lung volume testing by excluding restriction based on a normal VC.
QUICK LOOK
Current knowledge
Although current ATS/ERS PFT interpretation guidelines recommend using the largest measured vital capacity (VCmax), clinically, many continue to use forced vital capacity (FVC) due to convenience. Additionally, there is disagreement regarding whether the post-bronchodilator measurements of VC (post-bronchodilator FVC or even slow VC measured following bronchodilator) may be used in diagnosing obstruction.
What this paper contributes to our knowledge
In this study of clinical PFT interpretation, the vast majority of PFTs had the same interpretation regardless of whether the FVC or the largest measured VC, including post-bronchodilator values, VCmax, was used. However, in a minority of PFTs, using the VCmax for interpretation ruled out restriction or diagnosed obstruction, or both. This approach may be helpful in interpreting some PFTs where the diagnostic category is unclear.
Methods
Institutional review board approval was obtained from Intermountain Healthcare, and informed consent was waived for this retrospective, de-identified, data-only study.
Data Set
First, we developed an electronic decision support tool for PFT interpretation, eProtocol-PFT,8 based on current ATS/ERS guidelines.9 We validated the tool by using it in an open-loop manner with a pulmonologist accepting or declining the PFT interpretation generated by eProtocol-PFT. We iteratively modified the logic of eProtocol-PFT to fit with the ATS/ERS PFT interpretation guidelines.
Our de-identified clinical PFT database contained 12,238 PFTs collected as part of routine clinical care from consecutive visits at Intermountain Medical Center and LDS Hospital between October 15, 2001 and February 15, 2013. Our data set was limited to acceptable and reproducible spirometry curves as defined by ATS/ERS 2005 recommendations and included only white subjects, where the lower predicted limit guidelines are well-established.10 NHANES III prediction equations were used to calculated the lower predicted limit values for the FEV1/FVC, and, in our clinical data set, these were very similar to the more recent ERS/GLI12 predicted equations.11
eProtocol-PFT Interpretations
Using eProtocol-PFT, we analyzed all of the PFTs twice. First, we analyzed the PFTs using the pre-bronchodilator FEV1/FVC to determine obstruction and FVC as the VC in the algorithm. Next, we analyzed all PFTs and evaluated all VCs to identify the largest, VCmax. Obstruction was defined by an FEV1/VCmax that was lower than the lower predicted limit, where the FEV1 was the pre-bronchodilator FEV1, and the VCmax was the largest VC measured during the visit (including post-bronchodilator values). A normal VCmax excluded a diagnosis of restriction, in the absence of measurement of TLC by body plethysmography.
All PFT interpretations were classified by eProtocol-PFT in one of 9 possible categories: normal, obstruction, restriction, likely restriction, mixed obstruction and restriction, obstruction with possible restriction, nonspecific pattern,4 isolated abnormal FEV1 (all other spirometry values normal), and isolated abnormal FVC (all other spirometry values normal). These latter 2 categories have subsequently been classified to be included in the nonspecific pattern6; however, we kept them separate as per the original publication.4 An isolated FEV1 reduction may be quite different from an isolated FVC reduction, since reclassification of the FEV1/VCmax as reduced would quite clearly fit with mild obstruction in the former, whereas it would leave more room for uncertainty in the latter. Thus, we chose to keep them as separate categories. A positive bronchodilator response was defined by ATS/ERS criteria of an increase in FEV1 or FVC of ≥200 mL and 12%.9
Data Analysis
We determined what percentage of PFTs had a change in interpretation when VCmax was used rather than FVC. Then we classified the changes that occurred to determine in which categories the PFT interpretations were changed. We examined several factors related to subject demographics and PFTs to determine which factors were most likely to predict a change in interpretation when VCmax was used instead of FVC in the algorithm. We determined how often the source of the VCmax was the post-bronchodilator FVC and quantified the interpretive changes. We also determined the number of PFTs showing nonspecific pattern and quantified how often the nonspecific pattern was classified as obstruction when FEV1/VCmax was used in the interpretation.
Statistical Analysis
To determine the factors most likely to predict a PFT interpretive change, we examined a number of variables for differences in medians (with corresponding interquartile ranges) and proportions based on whether a PFT was recategorized. We used the Bonferroni correction to adjust for multiple comparisons. We then examined all variables for their predictive value of recommendations for recategorization using a logistic regression model reporting adjusted odds ratios. The model satisfying our diagnostic criteria, generating the highest C statistic (area under the curve), and achieving the most parsimonious solution was used. All analyses were performed in Stata 12 (StataCorp, College Station, Texas).
Results
Baseline Characteristics
We analyzed 12, 238 PFTs, from 9,916 unique subjects. Table 1 summarizes the baseline characteristics of the subjects, and Table 2 summarizes the baseline characteristics of the PFTs. Almost half of the PFT interpretations were normal, with nearly a quarter diagnosed with obstruction (Table 2). Restriction occurred in 4.4%, and possible restriction (where measurement of TLC by body plethysmography was not available) occurred in 12.4%. Mixed obstruction and restriction occurred in 0.9%, and obstruction with likely restriction (where TLC was not measured by body plethysmography) occurred in 8.1%. A bronchodilator test was performed 49% of the time (6,009 of 12,238). There was a positive bronchodilator response in 1,118 of 6,009 (18.6%) of PFTs when post-bronchodilator testing was performed.
Summary Descriptive Statistics by Change in Pulmonary Function Test Interpretation
Pulmonary Function Test Interpretation Frequency With FVC
Change in PFT Interpretation With VCmax
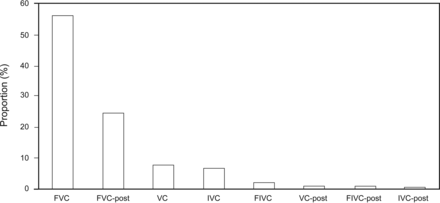
Interpretations changed in 710 of 12,238 (6%) of PFTs with VCmax (instead of FVC). The sources of the VCmax are summarized in Figure 1. The pre-bronchodilator FVC was the source of the VCmax the majority of the time (56.4%). When a post-bronchodilator test was performed, a post-bronchodilator VC was the source of the VCmax 55.7% of the time (3,346 of 6,009). Ninety-one percent of the time (3,045 of 3,346), that post-bronchodilator VC was the post-bronchodilator FVC. Overall, the post-bronchodilator FVC was the source of VCmax 24.9% of the time (3,045 of 12,238).
The source of the largest measured vital capacity is shown. FVC is the source of the largest measured vital capacity the majority of the time, followed by the post-bronchodilator FVC. FIVC = forced inspiratory vital capacity; IVC = inspriatory vital capacity.
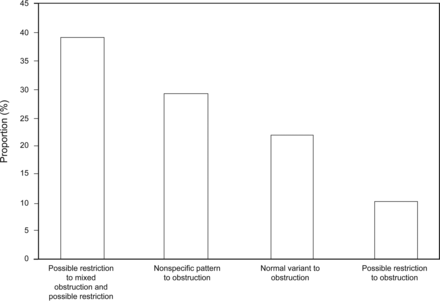
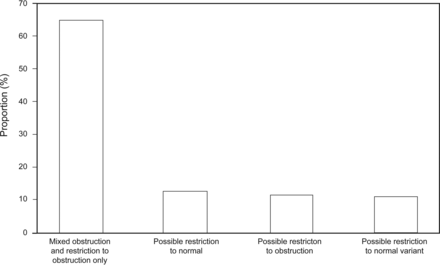
Two hundred forty-seven of 710 PFTs (35%) were reclassified with a new diagnosis of obstruction (Fig. 2). Possible restriction was reclassified to obstruction with possible restriction in 96 of 247 (39%). Seventy-two of 247 times (29%), the nonspecific pattern was reclassified to obstruction. A normal VCmax in 228 of 710 (32%) eliminated the “suggested restriction” in PFTs with a reduced FVC (Fig. 3). The cases (26 of 710, 3.7%) in which both restriction was ruled out and obstruction was diagnosed were included in both Figures 2 and 3.
Using the largest measured vital capacity (VCmax), 247 of 710 interpretations were recategorized as obstruction. The pulmonary function test interpretations that showed a new diagnosis of obstruction when VCmax was used are shown. Pulmonary function tests that were previously diagnosed as possible restriction, were recategorized as either obstruction (when VCmax was within predicted limits) or obstruction with possible restriction (when VCmax was reduced).
Using the largest measured vital capacity, 228 of 710 interpretations excluded restriction. Using the largest measured vital capacity, restriction was excluded in 228 PFTs. These were reclassified as obstruction or normal variants as shown.
Normal or normal variant PFTs were reclassified to obstruction in 54 subjects, or 22% (54 of 247) of the new obstruction diagnoses. Given that the predicted values for FEV1/VCmax used are derived from FEV1/FVC measurements in a normal population, it is unclear whether these cases represent an overdiagnosis of obstruction in our subjects or whether they identify subjects with disease.
Nonspecific Pattern Change in Interpretation
Three hundred sixty-six of 12,238 PFTs (3%) had the nonspecific pattern as defined by reduced FEV1 and FVC, normal FEV1/FVC, and normal TLC by body plethysmography. Seventy-two of 366 (20%) were reclassified as obstruction with VCmax.
Factors Predicting a Change in PFT Interpretation
We display the statistically relevant differences between all variables and the recommended change in recategorization with the exception of height (cm) and sex of the subject in Table 1. The most likely predictor of a change in PFT interpretation was the presence of a positive bronchodilator response (Table 3). The second most predictive factor leading to a change in PFT interpretation was having a post-bronchodilator spirometry test, even if the post-bronchodilator response was negative (see Table 3).
Factors Associated With Change in Pulmonary Function Test Interpretation Using Largest Measured Vital Capacity
A larger pre-bronchodilator FVC reduced the odds of a change in PFT interpretation. Surprisingly, the number of tests was not an independent predictor of a change in PFT interpretation. Thus, a subject who had pre-bronchodilator spirometry, diffusing capacity, and lung volume measurements was not more likely to have a change in PFT interpretation than a subject who only had pre-bronchodilator spirometry (Table 3).
Discussion
Although current guidelines recommend using VC in the interpretation of PFTs, the FVC continues to be frequently used due to convenience. In our large data set of clinical PFTs over more than a decade, 94% of the time, the PFT interpretation did not change with VCmax. However, in 6% of the PFTs, a change in PFT interpretation resulted, which resulted in clearer diagnostic categories for the PFTs when VCmax was used. The main changes were new diagnoses of obstruction and ruled out restriction, by virtue of a normal VCmax. The exclusion of restriction by a normal VCmax may have significant clinical implications in reducing further unnecessary diagnostic testing (eg, not adding TLC measurement by body plethysmography to the PFTs) and in increasing diagnostic accuracy. For example, diagnosing obstruction or normal variant in these cases of possible restriction has significant clinical implications. Additionally, in the cases where the PFTs fall in the nonspecific pattern or normal variant, a diagnosis of obstruction using FEV1/VCmax may be more clinically helpful in choosing treatment.
The fact that bronchodilator testing predicted PFT interpretation change, even if post-bronchodilator FVC was not significantly increased, suggests that obstruction may be more reflective of the underlying physiology of these subjects. For example, because these are clinical PFTs, clinicians probably have referred patients for bronchodilator testing if they suspect some obstructive lung disease. In these cases, increased variability in VC measurements or borderline values or both may result in a more likely diagnosis of obstruction with FEV1/VCmax, better reflecting the clinical problem.
Finally, ambiguity remains regarding whether post-bronchodilator FVC may be used when identifying the largest measured VC. Some have argued that the post-bronchodilator lung is in a different state, and thus it is improper to use the post-bronchodilator FVC with the pre-bronchodilator FEV1 to diagnose obstruction. However, there is broad agreement12 that if a positive bronchodilator response occurs, even if the post-bronchodilator FEV1/FVC remains normal (due to proportional increase in FEV1 and FVC), then obstruction is probably present and attributable to a volume response (an older term for which is pneumoconstriction).13 Our data support using the pre-bronchodilator FEV1/post-bronchodilator FVC to establish the diagnosis of obstruction in these cases. The simplicity and consistency of such an interpretive approach (pre-bronchodilator FEV1/post-bronchodilator FVC) may be easier for primary care providers and other consumers of PFT interpretations to use in clinical care.
Similarly, although the nonspecific pattern is accepted in the pulmonary community, many primary care providers are unaware of it and its implications. By using FEV1/VCmax, 20% of the nonspecific pattern PFTs may be interpreted as obstruction, which is a well-established category and fits in with the clinical correlates of airway hyper-reactivity reported for the nonspecific pattern.4
A major limitation of our study is the potential overdiagnosis of obstruction in otherwise asymptomatic people. Given the application of normal subject-derived FEV1/FVC predicted values to the FEV1/VCmax ratio in our clinical data set, it remains unclear how many of these cases are overdiagnoses of obstruction.
Conclusions
The vast majority of PFT interpretations were unchanged with VCmax. In 6% of the cases, there was a change in interpretation, and our data support evaluation of FEV1/VCmax in borderline or unclear cases, especially when normal variant or nonspecific pattern interpretations are present. This approach must be coupled with clinical correlation, given the concern for potential overdiagnosis of obstruction, but it may aid clinicians in diagnosing obstruction. When restriction is suspected due to a reduced FVC, examination of all measured VCs may aid clinicians in excluding restriction and avoiding additional testing and further clarify the diagnosis by evaluating whether obstruction (reduced FEV1/VCmax) is present. This diagnostic approach may help to evaluate ambiguous PFTs but also requires individual assessment of patient symptoms and complaints. Broader use of the VCmax instead of the FVC in routine clinical interpretation of PFTs will probably rely on incorporation of this algorithm into PFT machines and careful clinical correlation.
Acknowledgments
We are grateful to Steven Howe PhD, who was instrumental in helping us to obtain the PFT data in a usable form.
Footnotes
- Correspondence: Denitza P Blagev MD, Intermountain Medical Center, Heart-Lung Building, 6th Floor, 5121 South Cottonwood Street, Murray, UT 84107. E-mail: Denitza.blagev{at}imail.org.
This work was supported by a grant from the Intermountain Research and Medical Foundation. The authors have disclosed no conflicts of interest.
Dr Blagev presented a version of this paper at the American Thoracic Society 2013 International Conference, held May 17–22, in Philadelphia, Pennsylvania, and at the European Respiratory Society International Congress 2014, held September 6–10, 2014, in Munich, Germany.
- Copyright © 2016 by Daedalus Enterprises