Abstract
BACKGROUND: The systematic implementation of evidence-based practice through the use of guidelines, checklists, and protocols mitigates the risks associated with mechanical ventilation, yet variation in practice remains prevalent. Recent advances in software and hardware have allowed for the development and deployment of an enhanced visualization tool that identifies mechanical ventilation goal variance. Our aim was to assess the utility of daily goal establishment and a computer-aided visualization of variance.
METHODS: This study was composed of 3 phases: a retrospective observational phase (baseline) followed by 2 prospective sequential interventions. Phase I intervention comprised daily goal establishment of mechanical ventilation. Phase II intervention was the setting and monitoring of daily goals of mechanical ventilation with a web-based data visualization system (T3). A single score of mechanical ventilation was developed to evaluate the outcome.
RESULTS: The baseline phase evaluated 130 subjects, phase I enrolled 31 subjects, and phase II enrolled 36 subjects. There were no differences in demographic characteristics between cohorts. A total of 171 verbalizations of goals of mechanical ventilation were completed in phase I. The use of T3 increased by 87% from phase I. Mechanical ventilation score improved by 8.4% in phase I and 11.3% in phase II from baseline (P = .032). The largest effect was in the low risk VT category, with a 40.3% improvement from baseline in phase I, which was maintained at 39% improvement from baseline in phase II (P = .01). mechanical ventilation score was 9% higher on average in those who survived.
CONCLUSIONS: Daily goal formation and computer-enhanced visualization of mechanical ventilation variance were associated with an improvement in goal attainment by evidence of an improved mechanical ventilation score. Further research is needed to determine whether improvements in mechanical ventilation score through a targeted, process-oriented intervention will lead to improved patient outcomes. (ClinicalTrials.gov registration NCT02184208.)
- mechanical ventilation
- quality
- safety
- health-care quality
- ICU
- critical care
- computer decision support
- visualization of data
Introduction
The systematic implementation of evidence-based practice through the use of guidelines, checklists, and protocols has been shown to mitigate the risks associated with mechanical ventilation,1–19 yet variation in practice remains prevalent; this variation may be powered by conflicting evidence of the effectiveness of guidelines, checklists,20 and protocols21–24 to improve meaningful clinical outcomes. Recent advances in mechanical ventilation, physiologic monitoring, device-to-device communication, computer processing, and software engineering have allowed for the development and deployment of enhanced visualization systems that alert clinicians to practice variance. This may allow for easier determination of adherence in a wide variety of environments, cultures, and team makeups. Research has shown that automation is able to improve the quality and safety of care delivered by the health-care team.25–27 The increase in automation of care will probably continue as pressure from government agencies, purchasing groups, and consumers of health care demand accountability for variances from the goals or standards of care.28–31 As a result, most institutions are in a continuous state of technological improvement.
With the ongoing publication of medical research and the development of evidence-based clinical practice guidelines, clinicians are faced with challenges of reviewing, evaluating, and maintaining competency. Advances in mechanical ventilation over the last decade have resulted in improved monitoring, more established predictors for titrating support, and a better understanding of how lung injury occurs. There is evidence that adopting clinical practice guidelines together with lung-protective ventilation strategies reduces mortality in adult patients.32–36 However, we rarely evaluate adherence to established goals of mechanical ventilation in near real time.
The quality of mechanical ventilation is often based on outcomes such as mortality, length of hospital stay, duration of mechanical ventilation, ventilator-free days, ventilator-associated pneumonia, incidences of pneumothoraces, or other organ injury. These quality measurements are retrospective in nature and do not offer the clinician an opportunity to identify variances from established goals and standards of care in an effort to apply corrective action. Quality of mechanical ventilation should focus on but not be limited to: (1) point-of-care access to real-time variance identification of continuous data, (2) automatic recommendation of care needs, and (3) identification of and addressing of care gaps. These processes should result in fewer complications and a reduction in duration of mechanical ventilation, sedation dependence, stay, and associated cost of care. Our study attempts to evaluate the effects of point-of-care access to real-time variance identification and its effects on a locally developed mechanical ventilation score of overall performance. Therefore, we sought to examine the utility of a basic form of computer decision support, visualization of variance from goals of therapy in a cohort of children receiving mechanical ventilation.
QUICK LOOK
Current knowledge
Quality measurements of mechanical ventilation often include outcomes such as mortality, length of stay, time to liberation, ventilator-free days, ventilator-associated events, and/or incidence of ventilator-associated pneumonia. The cause of poor quality is often attributed to lack of adherence to the established goals or standards of care. Currently, only significant deviations from goals or standards of care are reported by intermittent assessments, such as root cause analysis, and these are often associated with undesirable outcomes. To our knowledge, there is no continuous real-time variance assessment tool to identify mechanical ventilation therapy other than manual retrospective analysis, such as chart review.
What this paper contributes to our knowledge
This study provides a process for establishing goals and real-time identification of variances through the use of a computer-aided mechanical ventilation monitoring system. Computer-aided mechanical ventilation monitoring reduced variances and improved goal attainment. This paper further describes the strengths and limitations of a computer-aided mechanical ventilation scoring system.
Methods
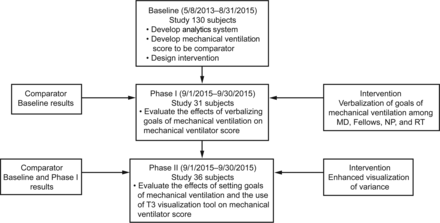
Following a 2.5-y system development and data collection period of baseline data, we conducted a 2-phase sequential intervention study. The baseline data collection period retrospectively scores the complete course of mechanical ventilation subjects within a single pediatric ICU to use as a historical control. Phase I sought to address the effects of verbalizing goals of mechanical ventilation daily on the mechanical ventilation score. Phase II was conducted to evaluate the cumulative effect of verbalizing goals of mechanical ventilation daily and setting goals within the T3 system that provides enhanced visualization of variance available on any computer within our hospital. See Figure 1 for an overall schematic presentation of phases.
Overall sequential schematic of phases. The baseline phase was used to develop the methods of data collection and scoring. Phase I was designed to test the effect of discussing goals of mechanical ventilation with the care providers. Phase II followed phase I to test the effect of enhanced visualization within the T3 system a minimum of 4 times/d. MD = physician, NP = nurse practitioner, RT = respiratory therapist.
Study Population
This single-center study at the Boston Children's Hospital medical surgical ICU consisted of critically ill pediatric subjects with medical and non-cardiac surgical diseases. The study population included subjects who required mechanical ventilation for >3 h in which continuous data were available. This study was approved by the Boston Children's Hospital investigational review board and the critical care medicine quality improvement committee.
Data Collection and Display System
Data collection was based on the availability of connectivity equipment to a data network that stores, analyzes, and displays information within the trending, tracking, and triggering system dubbed “T3” (Etiometry, Boston, Massachusetts). The primary functions of the T3 monitor are to aggregate, store, and display comprehensive real-time patient data for clinicians. The technology provides an FDA-cleared and HIPAA-compliant platform to track patient data on a single, easy-to-manipulate monitoring system, viewable on standard web browsers within the hospital infrastructure.
Data Handling of the Outcome Measurements
We partnered with a newly formed software and analytics development firm (Etiometry, Boston, Massachusetts) to further add functionality to the commercially available T3 system in the form of an analytics platform with the ability to formulate and collate multivariable algorithms for research purposes.
The computer-aided mechanical ventilation monitoring module is built on the T3 monitor platform. Mechanical ventilators stream their data through the critical care bedside monitor (MP90 or MX800, Philips Healthcare, Amsterdam, Netherlands) using a proprietary data connectivity engine by the same company labeled Intellibridge utilizing the EC-10 module and device-specific cables. The bedside critical monitor streams data into the Philips servers. The Philips servers continuously export HL7 standardized data to the T3 monitor server at a frequency of once every 5 s. Ideal body weight was calculated by the respiratory therapists, documented, and then extracted from the electronic medical record. For the purposes of this proof-of-concept study, the physiologic, mechanical ventilation, and ideal body weight data were combined manually with demographics and reason for mechanical ventilation. Eighty reasons for mechanical ventilation were grouped into 2 general cohorts: medical and surgical. No computer-aided mechanical ventilation outcome measurements were available to the clinicians at the time of the study with the exception of the commercially available T3 monitor in phase II.
Outcome Measurements
Subject Categorization.
Subject categorization was conducted as described previously by Walsh et al.37 In brief, subject categorization uses a system of rules-based IF-THEN algorithms covering 4 clinical domains of outcomes: (1) ventilation and (2) oxygenation and ventilator-induced lung injury (VILI) described as either (3) low risk PIP or (4) low risk VT. Table 1 provides the definitions of the rules-based algorithms by acceptable category. Acceptable categories and VILI indices were developed based on unit policy and guidelines. For categories not found in guidelines or policies, a modified Delphi method utilized the experts of our respiratory research group. The categorization algorithm calculated the patient status minute-by-minute and was manually run at the conclusion of the mechanical ventilation course. All categorization was calculated as a percentage of total duration of mechanical ventilation.
Rules-Based Algorithms and Definitions Used to Provide Subject Categorization Within the 3 Domains
Acceptable ventilation was defined as breathing frequency below the tachypnea classification for age and an PETCO2 of 35–55 mm Hg. Acceptable oxygenation was designed to utilize SpO2/FIO2 as reported previously by the Pediatric Acute Lung Injury Consensus recommendations38 and validated by Tripathi39 and Thomas et al.40 If the SpO2 was >97%, the SpO2/FIO2 was not calculated. If the SpO2/FIO2 was >264, the oxygenation was considered acceptable. Measurements of VILI were peak inspiratory pressure and exhaled tidal volume defined (VT exhaled/kg of ideal body weight); therefore, to negatively score measurements associated with VILI, we created a low risk PIP state defined as a peak inspiratory pressure ≤30 and low risk VT states defined as a VT exhaled/kg between 4 and 8 mL/kg.
Mechanical Ventilation Score.
Outcomes of acceptable mechanical ventilation were utilized to develop the mechanical ventilation score. This allowed us to develop a single outcome indicator. One hundred percent is the maximum score, meaning the complete mechanical ventilation course was spent within the standards of care. All algorithms and the mechanical ventilation score were developed a priori to the study. Twenty subjects were manually reviewed and scored to ensure the accuracy and intent of the mechanical ventilation score: Mechanical ventilation score = AV (0.25) + AO (0.25) + BF (0.25) + LRVT (0.25). The mechanical ventilation score utilizes acceptable oxygenation (AO) and acceptable ventilation (AV) status without VILI measurements, such as low risk PIP (LRP) or low risk VT (LRVT). All 4 categories were equally weighted and calculated for each minute of mechanical ventilation and summarized at liberation. The mechanical ventilation score was our primary outcome measurement to evaluate our interventions of establishing daily goals and enhanced visualization of variance from goals of mechanical ventilation.
Baseline.
Following the development of the outcome measurements and analytic platform, we retrospectively analyzed subjects who required invasive mechanical ventilation within our medical surgical ICU from May 8, 2013 to August 31, 2015. This cohort (baseline phase) was used as the comparator for the next 2 interventions.
Intervention: Phase I.
Daily rounds of subjects receiving mechanical ventilation were observed on 1 of 3 teams for weeks 1–4 (month of September 2015) by a single research respiratory therapist not involved in the subjects' care. Six goals of mechanical ventilation (heart rate, breathing frequency, SpO2, FIO2, PETCO2, and VT) were verbalized daily. When the information was not provided verbally during rounds, the researcher would ask for the missing goal of therapy. For subjects in whom direct observation could not be completed (ie, subjects on the 2 other teams not observed), the researcher would ask the physician, nurse practitioner, or respiratory therapist responsible for their care to verbalize the 6 goals of mechanical ventilation. Defaults established by clinical practice guidelines or policies were provided to those who asked.
Intervention: Phase II.
Following education on how to use the T3 monitor system, daily rounds of subjects receiving mechanical ventilation were observed on 1 of 3 teams for weeks 5–8 (month of October 2015) as conducted in phase I. Daily goals of mechanical ventilation were verbalized and entered into the T3 monitor. The T3 monitor provides enhanced visualization of the patient's physiologic and mechanical ventilation data and highlights parameters outside of goal (Fig. 2). The technology provides a platform to track vital patient data on a single easy-to-manipulate monitoring system. The T3 platform was utilized on rounds and during case conferences when subjects were discussed. Clinicians were simply asked to look for parameters that were shaded (variance state) for further investigation. T3 data were assessed at least twice per shift to augment clinical assessments. Initial set-up of the T3 goals takes about 4 min/patient. Daily review and adjustment of T3 takes <2 min to review for variances (shading) per patient.
An example of the T3 user interface when goals are outside of standard. Goals of mechanical ventilation are set on the right-hand side of the screen. The green shading represents a minute-by-minute evaluation of the heart rate above goal, the red shading represents a blood pressure below target, and the gray represents 3 epochs of the tidal volume being above goal. Shading is only present when a parameter is outside of goal.
The main T3 user interface (see Fig. 2) enables clinicians to explore a patient's physiologic measurements both as instantaneous values and as a time series. Data streams are displayed across the central screen, with all available data streams and goals shown in the right panel. Multiple data streams can be visualized simultaneously, arranged in a custom sequence, and sorted by user-defined overlays. The time window can be expanded or contracted for rapid visualization of a patient's current status or historical course up to 2 weeks. Goals of mechanical ventilation were specified to provide prompt visualization of anomalous or undesirable physiologic values.
Protocol Adherence.
To ensure research protocol adherence, the research respiratory therapist observed bedside rounds on 24 d of each of the 4-week periods. The T3 administrative function was used to record logins and duration of logins between phases. As part of the T3 education, an average increase of 4 logins/subject/day was the expected effect size.
Data/Statistical Analysis.
The T3 display of data is not filtered and is capable of zooming in on a visualized measure to a sample frequency of every 5 s. For retrospective categorization purposes, a 1-min median was applied to the 12 samples acquired within each minute at the conclusion of each mechanical ventilator course. From the 1-min median sample, a subject categorization was determined. Each subject categorization was calculated as a percentage (0–100%) of time spent within the condition. Zero means that no categorization was triggered, and 100% indicates that the complete mechanical ventilation course was spent within that category. Acceptable categorization was assumed if no other categorization was triggered. Continuous variables, means, and variances were evaluated using the Shapiro-Wilks test of normality and the Levene test of homoscedasticity. Means, medians, and 25–75% quartiles were calculated from the 1-min sampled parameters according to absolute number of measurements. One-way analysis of variance using the nonparametric Kruskal-Wallis test was performed on data that failed normality. Comparison of pairs was performed utilizing the nonparametric Wilcoxon method of analysis. Dichotomous variables were compared using the chi-square test. P values of <.05 were considered significant. We decided a priori that a >5% increase from baseline in phase I and a >10% increase from baseline in mean mechanical ventilation score would be considered clinically important. JMP 12.0 (SAS Institute, Cary, North Carolina) software was utilized for the statistics, categorization, and graphs.
Results
Demographics
Baseline.
A baseline sample of 130 subjects was analyzed to be the referent. The median age was 4 y, the median ideal body weight was 15 kg, and 58% of the subjects were male. The majority of subjects who required mechanical ventilation were categorized as surgical (53%), and the hospital mortality was 8.3%. Severity of illness by Pediatric Index of Mortality 3 score was 0.42. See Table 2 for details.
Subject Demographics and Mortality by Cohort
Phase I.
Phase I enrolled 31 subjects from September 1, 2015 to September 30, 2015. The median age was 7 y, the median ideal body weight was 23.4 kg, and 58% of the subjects were male. The majority of subjects who required mechanical ventilation were categorized as surgical (74%), and the hospital mortality was 11.5%. Severity of illness by Pediatric Index of Mortality 3 score was 0.34. See Table 2 for details.
Phase II.
Phase II enrolled 36 subjects from October 1, 2015 to October 31, 2015. The median age was 2.5 y, the median ideal body weight was 12.9 kg, and 56% of the subjects were male. The majority of subjects who required mechanical ventilation were categorized as surgical (58%), and there were no hospital mortalities. Severity of illness by Pediatric Index of Mortality 3 score was 0.32. See Table 2 for more details. (No significant differences were found in age, weight, race, sex, general classification of medical or surgical between cohorts, mortality, or severity of illness.)
Phase I Verbalization of Goals
A total of 171 verbalizations of mechanical ventilation goals were completed on the 31 subjects enrolled during 26 of the 31 calendar days, including 4 weekend days. Thirty-nine percent of the verbalization occurred during morning multidisciplinary rounds. The remaining 61% of the verbalizations were conducted by direct interview of the respiratory therapist, nurse practitioner, or physician caring for the subject.
Phase II Verbalization, Goal Entry, and Visualization of Variation Within T3
T3 Use.
The intervention to increase the use of T3 was successful. The use of T3 increased in duration by 74% from 139 min of use in phase I to 233.7 min/d in phase II. The number of logins increased by 87% from 5.9 logins in phase I to 10.8 logins/d in phase II.
Mechanical Ventilation Score.
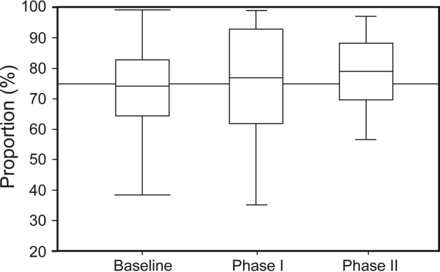
Mechanical ventilation score incrementally improved by 8.4% in phase I and 11.3% in phase II from baseline (P = .032), exceeding our clinical significant hypothesis by 3.4 and 1.3%, respectively. See Figure 3 for details. Further analysis demonstrates that the most significant change occurred between baseline and phase II with a P = .03. The largest individual category improvement was low risk VT with a 28% increase in phase I and continuation to an increase of 36% in phase II from baseline (P = .01). See Figure 4 and Table 3 for more details. There was a positive trend in the acceptable oxygenation category.
Box plot of mechanical ventilation score by phase. Boxes represent 25–75% intervals, center lines denote the median, and whiskers show 10–90% intervals.
Radar chart of the patient categories that make up mechanical ventilator score by phase. The dotted lines represent the phases and percentages of time spent within the acceptable categories. The closer the line is to the outside category, the more acceptable (100%). The different colors represent the differences between phases of the study. Mechanical ventilator score and low risk VT categories were the only significant findings. * Statistically significant results using the Wilcoxon method of analysis. There was a trend toward a higher percentage of acceptable oxygenation, but it did not reach statistical significance.
Subject Categorization Results by Phase
Overall Performance of Mechanical Ventilation Score
The mechanical ventilation score was 9% higher on average in those who survived (76%, interquartile range 67.5–85.9%) than in those who died (69.7%, interquartile range 35–78.1%) (P = .02) (Fig. 5A). We calculated the probability of ICU survival by mechanical ventilation score percentage. Figure 5B illustrates probability estimated based on observed results. The mechanical ventilation score did not correlate with ICU length of stay, hospital length of stay, or mechanical ventilation length of stay when including all patients. However, when examining the surgical cohort by those who scored ≥70%, we found a shorter length of hospital stay (log-rank P = .042, Fig. 6A) and a lower mortality (likelihood P < .001, Fig. 6B).
A: Box plot of mechanical ventilation score by survival among all subjects. B: Probability plot of survival based on mechanical ventilation score. The two outer lines represent 95% CI.
A: Kaplan-Meier estimate of hospital length of stay among surgical subjects. The red area represents the cohort that scored <70%, and the blue represents the cohort that scored >70%. B: mosaic plot of a contingency analysis of survival among surgical subjects who scored <70% or >69%.
Parameters and Calculations
Three parameters were significantly improved with the interventions. FIO2 was reduced from baseline by 5.3% in phase I and continued to decrease by 8% in phase II (P = .02). Peak inspiratory pressure decreased from baseline by 16.5% in phase I and maintained the same improvement in phase II (P < .001). PEEP was lower statistically (P = .031) but not clinically significantly (Table 4).
Parameters of Oxygenation, Ventilation, Mechanical Ventilation Pressures, and Duration by Phase
Discussion
We demonstrate the use of a method that establishes and verbalizes daily goals and provides enhanced visualization of mechanical ventilation variance by using a computer-aided system. Our recently developed analytic platform allows us to examine our interventions and share the results with our staff. This method provides enhanced visualization in real time followed by an objective measure of the standard of care designed to reduce and evaluate variance in mechanical ventilation practice. Although we expected a cumulative effect of each intervention, surprisingly almost half (44%) of the largest improvements were seen in phase I with verbalization of goals of mechanical ventilation. The power of clear communication of goals of mechanical ventilation should not be underestimated and can independently improve goal attainment. This must be considered with the development of any computer decision support system.
The visualization of variance using the T3 monitor was considered helpful by the staff. However, some found it hard to estimate how long the subject had been out of variance or confusing when multiple parameters were outside of target. This led to speculation that if a continuous calculation of the mechanical ventilation score had been provided, goal attainment would have been better. We hope to further develop the system to offer more real-time analysis of the mechanical ventilation score.
Visualization of Mechanical Ventilation Variance
Display of variance for all to see makes managing these patients a team effort. A significant amount of human filtering is done when verbalizing and documenting assessments; therefore, when visualization tools within higher end electronic health records are utilized, their relevance is often questioned. Additionally, goals of therapy are often not utilized to demonstrate variance. Our electronic health record uses “generally accepted norm” to identify vital signs outside of what they consider normal, but there is no determinate of time spent outside of goal. There are no such generally accepted norms for mechanical ventilation within our electronic health records.
Mechanical Ventilation Score
Health care is shifting the focus from the volume of mechanical ventilation services provided without major complications to the value created for patients/families. Value is being defined as outcomes achieved relative to cost.41 Progress has been slow because outcomes that matter to patients and families, aside from survival, remain limited.42 The outcome of death in pediatrics is fairly rare, which makes this measure difficult to use to separate excellence from competence. If we were to truly unlock value-based mechanical ventilation, we must commit to measuring a minimum set of outcomes for every major medical disease that requires this therapy.
The mechanical ventilation score proposed in this study was the first attempt at quantifying goals of mechanical ventilation into a single score as an outcome for research purposes. The mechanical ventilation score is a minute-by-minute assessment not humanly possible in current practice. This continuous assessment has the potential to identify and quantify variances not previously seen before with intermittent assessments. Since the mechanical ventilation score is not a validated score of mechanical ventilation quality, you will notice that we refrain from indicating such. However, it is plausible that our domains of surveillance (oxygenation, ventilation, VILI) in addition to traditional metrics are important aspects of mechanical ventilation therapy and could be used to determine the quality of mechanical ventilation.
A higher mechanical ventilation score was associated with a lower mortality among all subjects scored. A lower mechanical ventilation score utilizing a cutoff score of 70% was associated with a longer length of hospitalization within our surgical cohort. This finding did not hold true when applied to all subjects. It is difficult to speculate whether poor quality mechanical ventilation or severity of illness led to the higher length of stay. Although pediatric patients may be less susceptible to ventilator-induced lung injury,43 we may discover cohorts of patients in which small periods of ventilation outside of accepted ranges may be clinically important, as we have described in our surgical population. Future research will require the exploration of the effect of mechanical ventilation score on meaningful outcomes. If there is a relationship, providing a real-time mechanical ventilation score may give clinicians the opportunity to intervene and change the trajectory of the patient's course.
Hawthorne Effect
Although we speculate that a positive effect from this study was the result of verbalization of goals and enhanced visualization of variance, we cannot rule out the Hawthorne effect,44–46 since clinicians were keenly aware we were collecting data. The surveillance process required the clinicians to think about and know the subjects' daily goals. Since the establishment and verbalization of goals was identical between phase I and phase II, the differences are the result of the visualization of variance from stated goals.
Disease-Specific Goals
We did not develop disease- or patient-specific outcome measurements of mechanical ventilation, and that clearly needs to be a next step in our program's progression. We set out to determine the feasibility of our interventions utilizing broad goals applicable to the majority of our mechanically ventilated patients. There are some patients who may require a non-traditional approach to mechanical ventilation, and this study was not designed to account for or assess them. For example, we prefer to keep the peak inspiratory pressure at <25 cm H2O in our patients who are mechanically ventilated for a congenital diaphragmatic hernia. Within our current system, it would only be considered barotrauma if the peak inspiratory pressure reached ≥30 cm H2O. VT of >8 mL/kg may be required in a patient with asthma to allow for prolonged expiratory time or in the neuromuscular diseased patient to generate enough flow to adequately clear secretions; however, within this protocol, this would have been counted minute by minute as not being within the standard of care.
When we were developing the study, we decided a priori that an increase of 5 and 10% improvement for phase I and phase II from baseline, respectively, would be a clinically important benefit of this system. Since our institution was going to implement this system as a standard of practice without study, we did not have the option to randomize or a protracted implementation process, which was a limitation in our study design.
Data Filtering
The T3 visualization system does not apply its own filters and reports only what the device sends. Ventilator disconnects and suctioning events are highlighted as a variance from goal. There is a notes section in which events such as turning, suctioning, or other events can be documented; however, this was rarely done. Clinicians investigating events would contextualize by noticing characteristics of events. For example, endotracheal tube suctioning events were typically preceded by an increase in FIO2 and SpO2 probe, or electrocardiogram lead off events were abrupt and followed by several seconds or minutes of no data because the monitor would not report a heart rate. We plan to work on algorithms to suggest events by combining the alarms and measured values in an effort to filter optimally. This is a current limitation of this system as it currently stands.
Data were collected at a frequency of once every 5 s (12 samples/min) and filtered to a 1-min median using a function built within the analytics platform before subject categorization and scoring. Each of the devices used typically has its own filtering capability to prevent the reporting of questionable measurements, but neither is perfect, and they occasionally report low or zero results during ventilator disconnects or lead/probe off events. The median method utilized within this project was our first step at reducing noise, but it is not perfect and is a limitation. The use of 1,440 filtered samples within a day as opposed to an average of 24 nursing and respiratory therapy documentations in a critically ill patient probably provides a superior assessment of variance. Such speculations have not been proven, and this represents another arena of research needed.
Conclusions
The process of surveying goals of mechanical ventilation and providing enhanced visualization of variances within a point-of-care system improves goal attainment, as evidenced by an institutional developed mechanical ventilation score. The mechanical ventilation score improved by 8.4% in phase I and 11.3% in phase II from baseline and exceeded our clinically relevant expectations by 3.4 and 1.3%, respectively. Further research is needed to determine whether improvements in mechanical ventilation score through a targeted, process-oriented intervention will lead to better patient outcomes and fewer medical errors.
Acknowledgment
We acknowledge the support and assistance of the respiratory therapists, physicians, and nurses in the medical surgical ICU of Boston Children's Hospital as well as Drs Beckett, Becker, and Kuperman for assistance and support by serving on Dr Walsh's dissertation committee.
Footnotes
- Correspondence: Brian K Walsh PhD RRT-NPS RPFT AE-C FAARC, Department of Anesthesiology, Perioperative and Pain Medicine, Division of Critical Care Medicine, Boston Children's Hospital, 300 Longwood Avenue, Bader 634, Boston, MA 02115.
Dr Rettig, Dr Walsh, Mr Smallwood, and Dr Arnold have received research support from Draeger Medical. Dr Walsh has received research support from GE Healthcare. Dr Kacmarek is a consultant for Covidien and OrangeMed and has received research grants from Covidien and Venner Medical. Mr. Thompson has disclosed no conflicts of interest.
See the Related Editorial on Page 382
- Copyright © 2017 by Daedalus Enterprises