Abstract
BACKGROUND: Spirometric Z-scores from the Global Lung Initiative (GLI) rigorously account for age-related changes in lung function and are thus age-appropriate when establishing spirometric impairments, including a restrictive pattern and air-flow obstruction. However, GLI-defined spirometric impairments have not yet been evaluated regarding associations with static lung volumes (total lung capacity [TLC], functional residual capacity [FRC], and residual volume [RV]) and gas exchange (diffusing capacity).
METHODS: We performed a retrospective review of pulmonary function tests in subjects ≥40 y old (mean age 64.6 y), including pre-bronchodilator measures for: spirometry (n = 2,586), static lung volumes by helium dilution with inspiratory capacity maneuver (n = 2,586), and hemoglobin-adjusted single-breath diffusing capacity (n = 2,508). Using multivariable linear regression, adjusted least-squares means (adjLSMeans) were calculated for TLC, FRC, RV, and hemoglobin-adjusted single-breath diffusing capacity. The adjLSMeans were expressed with and without height-cubed standardization and stratified by GLI-defined spirometry, including normal (n = 1,251), restrictive pattern (n = 663), and air-flow obstruction (mild, [n = 128]; moderate, [n = 150]; and severe, [n = 394]).
RESULTS: Relative to normal spirometry, restrictive-pattern had lower adjLSMeans for TLC, FRC, RV, and hemoglobin-adjusted single-breath diffusing capacity (P ≤ .001). Conversely, relative to normal spirometry, mild, moderate, and severe air-flow obstruction had higher adjLSMeans for FRC and RV (P < .001). However, only mild and moderate air-flow obstruction had higher adjLSMeans for TLC (P < .001), while only moderate and severe air-flow obstruction had higher adjLSMeans for RV/TLC (P < .001) and lower adjLSMeans for hemoglobin-adjusted single-breath diffusing capacity (P < .001). Notably, TLC (calculated as FRC + inspiratory capacity) was not increased in severe air-flow obstruction (P ≥ .11) because inspiratory capacity decreased with increasing air-flow obstruction (P < .001), thus opposing the increased FRC (P < .001). Finally, P values were similar whether adjLSMeans were height-cubed standardized.
CONCLUSIONS: A GLI-defined spirometric restrictive pattern is strongly associated with a restrictive ventilatory defect (decreased TLC, FRC, and RV), while GLI-defined spirometric air-flow obstruction is strongly associated with hyperinflation (increased FRC) and air trapping (increased RV and RV/TLC). Both spirometric impairments were strongly associated with impaired gas exchange (decreased hemoglobin-adjusted single-breath diffusing capacity).
- spirometry
- Global Lung Initiative
- static lung volumes
- restriction
- hyperinflation
- air trapping
- diffusing capacity
Introduction
In 2005, a combined task force of the American Thoracic and European Respiratory Societies (ATS/ERS) concluded that a restrictive ventilatory defect cannot be established by a spirometric restrictive pattern.1 The task force cited two studies2,3 in which a spirometric restrictive pattern, defined by a normal ratio of FEV1 to FVC but with low FVC, was associated with a restrictive ventilatory defect, defined by a low total lung capacity (TLC), “no more than half the time.”1 In the cited studies, diagnostic thresholds for spirometric measures and TLC were based on the lower limit of normal, defined as the fifth percentile of the distribution of reference values.2,3
The studies cited by the ATS/ERS task force have limitations regarding the lower limit of normal. For spirometry, the lower limit of normal was calculated from reference equations4–7 that incorrectly assumed a linear relationship between predictor variables (age and height) and spirometric measures, as well as incorrectly assumed a normal distribution and constant variability for reference values.8,9 For TLC, the lower limit of normal was calculated from reference equations that had small sample sizes, had low representation from older persons and non-whites, and were subject to a cohort effect (assembled before 1982).1,10,11 In addition, prior and current reference equations for TLC are often derived from samples drawn from populations that are different from those for spirometric reference equations, resulting in different predicted values for FVC (establishes spirometric restrictive pattern) versus slow vital capacity (used for calculating TLC).1,2,5–7,10,11
Subsequent to the 2005 ATS/ERS task force, an alternative approach was developed in 2008, termed lambda, mu, sigma,8 wherein the spirometric lower limit of normal (fifth percentile of distribution) was defined by a Z-score of −1.64. Lambda, mu, sigma–calculated Z-scores rigorously account for age-related changes in lung function, including variability in spirometric performance, by incorporating 3 elements of the distribution: skewness (lambda), median (mu), and coefficient-of-variation (sigma).8 In 2012, the Global Lung Initiative (GLI) expanded the availability of lambda, mu, sigma–calculated Z-scores by publishing reference equations that included ages up to 95 y and multiple ethnic groups.9 Clinically, Z-scores are routinely used in bone mineral density testing, and lambda, mu, sigma is widely applied to growth charts.8,12 Moreover, when established by lambda, mu, sigma–calculated and, more recently, by GLI-calculated Z-scores, normal spirometry and spirometric impairments, including restrictive pattern and air-flow obstruction, have strong phenotypic validation based on associations with health outcomes and radiographic imaging.13–19
Accordingly, to further evaluate restrictive ventilatory defects, but also establish broad physiologic validation, we have calculated adjusted least-squares means (adjLSMeans) for static lung volumes and diffusing capacity, expressed with and without height-cubed standardization20 and stratified by GLI-defined spirometric categories. Our analytical sample was large, including 2,586 subjects aged 40–93 years. We hypothesized that GLI-defined spirometric impairments would be associated with restrictive and obstructive ventilatory defects (static lung volumes) and impaired gas exchange (diffusing capacity), relative to GLI-defined normal spirometry.
QUICK LOOK
Current knowledge
Spirometric impairments (restrictive-pattern and airflow-obstruction), as defined by Z-scores from the Global Lung Initiative (GLI), have not yet been evaluated regarding associations with static lung volumes and diffusing capacity. In particular, it remains to be established whether GLI-defined spirometric restrictive pattern is associated with a restrictive ventilatory defect, whether GLI-defined airflow-obstruction is associated with hyperinflation and air trapping, and whether either spirometric impairment is associated with impaired gas exchange.
What this paper contributes to our knowledge
Using GLI-calculated spirometric Z-scores and based on comparisons with normal spirometry, we found that spirometric restrictive pattern was strongly associated with a restrictive ventilatory defect, including reductions in several static lung volumes (decreased total lung capacity, functional residual capacity, and residual volume), while spirometric airflow obstruction was strongly associated with hyperinflation (increased functional residual capacity) and air trapping (increased residual volume and the ratio of residual volume to total lung capacity). Both spirometric impairments were also associated with impaired gas exchange (decreased diffusing capacity).
Methods
Study Population
We reviewed pulmonary function tests (PFTs) on 2,586 subjects (1 PFT per subject) who were previously evaluated at the Veterans Affairs Connecticut Healthcare System and the Yale-New Haven Hospital. Specifically, of the 2,586 PFTs, 2,579 (99.7%) were reviewed consecutively, including 1,379 from the Veterans Affairs Connecticut Healthcare System over the period 2009–2012 and 1,200 from Yale-New Haven Hospital over the period 2013–2014. In addition, due to inadvertent time lapses, we reviewed 7 (0.27%) non-consecutive PFTs, including 4 from the Veterans Affairs Connecticut Healthcare System and 3 from Yale-New Haven Hospital, extending the time periods to 2008–2014 and 2013–2015, respectively.
In assembling our analytical sample, we required that PFTs were from subjects who were ≥40 y old and who self-reported white or black race at the time of the PFT visit and concurrently completed spirometry and static lung volumes. We additionally evaluated diffusing capacity, as this was almost always available. The inclusion of an age range of only ≥40 y was because age-related changes in lung function and the occurrence of chronic respiratory disease are more prevalent in middle-aged or older persons.8,9,18,19 The inclusion of only white or black race was because ethnic groups such as Asians and Hispanics comprised <2% of the available PFT sample population. The institutional review boards from Veterans Affairs Connecticut Healthcare System and Yale-New Haven Hospital approved the study.
Pulmonary Function Tests
At both the Veterans Affairs Connecticut Healthcare System and Yale-New Haven Hospital, PFTs included spirometry, static lung volumes by helium dilution, and single-breath diffusing capacity, collected by certified staff using ATS/ERS protocols and meeting ATS/ERS quality criteria.1,21,22–24
The spirometric measures of interest included pre-bronchodilator values for FEV1 and FVC, with FEV1/FVC calculated from the largest FEV1 and FVC that were recorded in any of the accepted spirometric maneuvers.1,22 The use of pre-bronchodilator values had two advantages. First, older persons have limited capacity to perform multiple FVC maneuvers (pre- and post-bronchodilator) and may experience an adverse response to a bronchodilator.25,26 Second, post-bronchodilator values have limited clinical relevance in distinguishing COPD from asthma and have low reproducibility over time.27
Using GLI reference equations,9 Z-scores were calculated for FEV1, FVC, and FEV1/FVC. The spirometric lower limit of normal was established by a Z-score of −1.64,8,9 with normal spirometry defined by FEV1/FVC ≥ lower limit of normal and FVC ≥ lower limit of normal, restrictive-pattern defined by FEV1/FVC ≥ but FVC < lower limit of normal, and air-flow obstruction defined by FEV1/FVC < lower limit of normal.13 The severity of air-flow obstruction was stratified according to previously validated FEV1 Z-score thresholds: ≥−1.64 as mild, <−1.64 but ≥−2.55 as moderate, and < −2.55 as severe.13,18,28,29
The static lung volumes included TLC, functional residual capacity (FRC), and residual volume (RV),23,24 also measured as pre-bronchodilator values. The testing protocol first established the FRC by single-breath helium dilution, with the end point defined by the helium concentration reaching equilibrium (change <0.02% for 30 s).23 This was followed by the inspiratory capacity and slow expiratory vital capacity maneuvers, respectively, with the inspiratory capacity maneuver linked to the FRC determination and with a best effort to establish the reported inspiratory capacity volume. The TLC was then calculated as FRC + inspiratory capacity, and the RV was calculated as TLC − slow vital capacity.
The single-breath diffusing capacity included values that were unadjusted as well as values adjusted for hemoglobin. Both were available in 97% of our analytical sample and were also measured as pre-bronchodilator values. The maneuver for the single-breath diffusing capacity began with unforced exhalation to RV, followed by a rapid inhalation to TLC.21
Reference Equations
We used GLI reference equations to establish normal spirometry and spirometric impairments, including the severity of air-flow obstruction.9 The GLI equations apply a rigorous, age-appropriate methodology and are based on large sample populations that include older age groups and minority representation.8,9
For reasons discussed earlier, we did not use reference equations for static lung volumes; similar reasons precluded the use of reference equations for diffusing capacity.10,21 Instead, values for static lung volumes and diffusing capacity were standardized to height-cubed. Prior work has shown that height-cubed standardization is associated with lung function over time and mortality, and may partially account for sex and size differences in lung function.20 In addition, adjLSMeans for static lung volumes and diffusing capacity were calculated across GLI-defined spirometric categories, using multivariable linear regression models and established predictors of lung function as control variables (potential confounders).8,9
Statistical Analysis
The demographic and anthropometric characteristics, smoking status, and PFT results were first summarized as means ± SD) or as counts and percentages. The PFT results additionally included measures standardized to height-cubed.
Next, multivariable linear regression models calculated unadjusted least-squares means and adjLSMeans for TLC, FRC, RV, RV/TLC, single-breath diffusing capacity, and hemoglobin-adjusted single-breath diffusing capacity, expressed in L or mL/min/mm Hg, as well as standardized to height-cubed. The main explanatory variable of spirometric function was stratified according to GLI-defined normal spirometry, restrictive-pattern, and the 3-level severity of air-flow obstruction (mild, moderate, and severe), respectively. The adjusted models included the covariates of age, height, sex, race, body mass index, and never-smokers. However, height was omitted in adjusted models that included measures standardized to height-cubed. Model goodness-of-fit was assessed by residual analysis and regression diagnostic measures; in models for which model fit was not entirely adequate, a robust variance estimator was used. Missing data for the static lung volume outcomes were minimal (<1%) and only slightly greater for diffusing capacity outcomes (3%); complete case analyses were therefore conducted.
Because prior work suggests a spirometric restrictive pattern is associated with a low TLC “no more than half the time,”1 the Pearson correlation of the FVC Z-score with TLC was evaluated within the category of a GLI-defined normal FEV1/FVC, which included both normal and restrictive-pattern. The TLC was expressed in L and standardized to height-cubed.
In addition, because TLC is calculated from the directly measured inspiratory capacity,23,24 and because progressively severe air-flow obstruction may limit the inspiratory capacity maneuver,30,31 the Pearson correlation of the FEV1 Z-score with inspiratory capacity was evaluated within the category of a GLI-defined decreased FEV1/FVC, which included only air-flow obstruction. The inspiratory capacity was expressed in L and standardized to height-cubed.
Results were interpreted as statistically significant if P values were <.05 for 2-sided tests. SAS version 9.4 software was used for all analyses.
Results
Table 1 shows demographic and anthropometric characteristics of the analytical sample (N = 2,586); mean age was 64.6 y, 27.8% were female, 86.4% were white, 13.6% were black, mean height was 170.0 cm, and mean weight was 88.8 kg (47.9% were obese). Regarding smoking status and spirometric categories, 23.1% were never-smokers, 53.8% were former smokers, 23.2% were current smokers, and 51.6% had GLI-defined spirometric impairment, including restrictive pattern in 25.6% and air-flow obstruction in 26.0%. Of those with air-flow obstruction (n = 672), 58.6% were severe. Table 1 also shows mean values for static lung volumes and diffusing capacity, expressed in L or mL/min/mm Hg and standardized to height-cubed.
Baseline Characteristics of the Study Sample
Tables 2 through 4 include unadjusted least-squares means and adjLSMeans for static lung volumes and diffusing capacity, consistently comparing GLI-defined spirometric impairments with GLI-defined normal spirometry. The reported results yielded similar P values regardless of whether the static lung volumes and diffusing capacity were standardized to height-cubed.
Least-Squares Means for Total Lung Capacity and Functional Residual Capacity
Least-Squares Means for Residual Volume and Residual Volume/Total Lung Capacity
Least-Squares Means for Diffusing Capacity
Table 2 shows results for TLC and FRC. Relative to normal spirometry, restrictive-pattern spirometry had lower adjLSMeans for TLC and FRC (P < .001), whereas mild and moderate air-flow obstruction had higher adjLSMeans for TLC and FRC (P < .001). For severe air-flow obstruction, only the adjLSMean for FRC, but not for TLC, was higher than normal spirometry (P < .001 for FRC and P values of .14 and .11 for TLC).
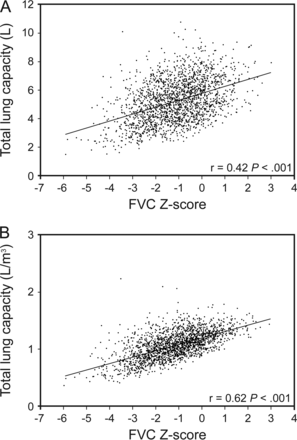
Figure 1 further evaluates the TLC based on FVC Z-scores in subjects with a GLI-defined normal FEV1/FVC (includes normal and restrictive-pattern). Figure 1 shows a significant linear correlation between a decrease in FVC Z-score and a decrease in TLC, especially when the latter is standardized to height-cubed (P < .001).
Pearson correlations of the FVC Z-score with the TLC for participants with a Global Lung Initiative-defined normal FEV1/FVC ≥ lower limit of normal (n = 1,914). Using Global Lung Initiative equations and the lower limit of normal at a Z-score of −1.64, a normal FEV1/FVC was defined by a Z-score ≥ −1.64. TLC, total lung capacity.
Figure 2 further evaluates a subdivision of TLC, specifically the inspiratory capacity, based on FEV1 Z-scores, in those with a GLI-defined decreased FEV1/FVC (includes only air-flow obstruction). Figure 2 shows a significant linear correlation between a decrease in FEV1 Z-score (denoting more severe air-flow obstruction) and a decrease in inspiratory capacity, especially when the latter is standardized to height-cubed (P < .001). Hence, in subjects with severe air-flow obstruction, the earlier result in Table 2 of the TLC not increasing (P values of .14 and .11) despite an increased FRC (P < .001) was due to a decreased inspiratory capacity (TLC is calculated as FRC + inspiratory capacity).
Pearson correlation of the FEV1 Z-score with the inspiratory capacity for participants with Global Lung Initiative-defined spirometric air-flow obstruction (n = 672). Using Global Lung Initiative equations and the lower limit of normal at a Z-score of −1.64, spirometric air-flow obstruction was defined by FEV1/FVC< lower limit of normal, with severity established by FEV1 Z-scores as mild (FEV1 Z-score ≥ −1.64), moderate (FEV1 Z-score < −1.64 ≥ −2.55), and severe (FEV1 Z-score < −2.55).
Table 3 shows results for RV and RV/TLC. Relative to normal spirometry, restrictive-pattern had a lower adjLSMean for RV but a higher for RV/TLC (P ≤ .001). Conversely, relative to normal spirometry, moderate and severe air-flow obstruction had higher adjLSMeans for both RV and RV/TLC (P < .001). For mild air-flow obstruction, only the adjLSMean for RV was higher than normal spirometry (P < .001); RV/TLC was otherwise similar (P = .11).
Table 4 shows results for single-breath diffusing capacity and hemoglobin-adjusted single-breath diffusing capacity. Relative to normal spirometry, restrictive-pattern and moderate and severe air flow-obstruction had lower adjLSMeans for single-breath diffusing capacity and hemoglobin-adjusted single-breath diffusing capacity (P < .001). For mild air-flow obstruction, however, the adjLSMeans for single-breath diffusing capacity and hemoglobin-adjusted single-breath diffusing capacity were similar to those in normal spirometry (P values ranged from .37 to .44).
Discussion
Using GLI-calculated spirometric Z-scores and based on comparisons with normal spirometry, our results show that a spirometric restrictive pattern identifies a restrictive ventilatory defect characterized by statistically significant lower values for TLC, FRC, and RV. Moreover, a restrictive pattern was associated with impaired gas exchange, as evidenced by a statistically significant lower value for hemoglobin-adjusted single-breath diffusing capacity.
We cannot confirm, however, that the association of a GLI-defined spirometric restrictive pattern with a lower TLC implies that this spirometric pattern will always have a low TLC (<lower limit of normal). As discussed earlier, reference equations to accurately calculate the lower limit of normal for TLC are unavailable. Nonetheless, because the objective is to assess restrictive physiology, the distinction between lower versus low TLC in the setting of a GLI-defined spirometric restrictive pattern may not be meaningful for 3 reasons. First, a lower TLC (and lower FRC and RV) in a GLI-defined spirometric restrictive pattern can only result from restrictive physiology. Second, a lower TLC (and lower FRC and RV) in a GLI-defined spirometric restrictive pattern is established relative to GLI-defined normal spirometry, which is known to have a normal respiratory phenotype, eg, physiological (normal FEV1/FVC and FVC), clinical (normal dyspnea grade, 6-min walk distance, and health-related quality of life), and by chest computed tomography.8,9,19 Third, a GLI-defined spirometric restrictive pattern has strong validation based on other measures, including an age-appropriate low FVC (GLI-calculated Z-score < −1.64 [lower limit of normal]) and known associations with multiple health outcomes across multiple cohorts.8,9,13–18,29
We further consider that the lower limit of normal threshold for establishing a low TLC may have diagnostic limitations regarding a restrictive ventilatory defect. Specifically, in established interstitial and chest-wall restrictive disorders, prior work has shown that TLC may be normal when FVC is decreased; conversely, a low TLC with normal FVC is rare.10 Hence, we propose an alternative physiologic definition of a restrictive ventilatory defect that is based on a GLI-defined restrictive pattern. As discussed earlier, a GLI-defined restrictive pattern has strong physiologic and clinical validation, and, as shown in Figure 1, the GLI-calculated FVC Z-score (criterion for distinguishing restrictive-pattern from normal spirometry) is strongly correlated with TLC, in a pattern consistent with a continuous function rather than a threshold effect.
Accordingly, simple spirometry (even a hand-held spirometer) could serve as a readily available tool for establishing a restrictive ventilatory defect. The latter is currently established by TLC in static lung-volume testing performed with gas-dilution techniques or whole-body plethysmography,1 thus requiring staff with specialized training and imposing greater cost and time.10 In addition, there are diagnostic limitations to the use of a lower limit of normal threshold for TLC.10
An issue that is not addressed by this study is the underlying mechanism of a GLI-defined spirometric restrictive pattern. In middle-aged or older persons, the list of potential restrictive disorders can include osteoporotic kyphosis, obesity, respiratory muscle weakness, pleural and interstitial lung diseases, heart disease, or pulmonary hypertension.32–39 Hence, in the absence of identifying the underlying mechanism, we could not determine whether the associations between a GLI-defined spirometric restrictive pattern and static lung volumes differed across the various restrictive disorders.
Notably, our study has also shown that a GLI-defined spirometric restrictive pattern is associated with a statistically significant higher value for RV/TLC, as compared with GLI-defined normal spirometry. Although a higher RV/TLC suggests increased air trapping, this is unlikely to be the case with the spirometric restrictive pattern. In prior work, using the same Z-score thresholds, a GLI-defined spirometric restrictive pattern was not associated with computed tomography–measured air trapping.18 Therefore, in our study, the higher RV/TLC in restrictive-pattern likely reflected a greater reduction in TLC. We note, however, that in a restrictive disorder caused by respiratory myopathy but with no underlying lung disease, the higher RV/TLC can additionally include a higher RV from expiratory muscle weakness.39
The physiology of GLI-defined spirometric air-flow obstruction is likewise informed by our study. In particular, statistically significant higher values for FRC (hyperinflation) and for RV and RV/TLC (air trapping) were found across the severity of air-flow obstruction. In contrast to FRC, however, we found that only mild and moderate GLI-defined air-flow obstruction had statistically significant higher values for TLC, relative to GLI-defined normal spirometry. Additionally, within the air-flow obstruction group, TLC declined across mild, moderate, and severe air-flow obstruction. Because inspiratory capacity and FRC are directly measured and combined to calculate TLC, and because our results show that inspiratory capacity decreases while FRC increases in severe air-flow obstruction, it follows that the decline in TLC across the severity of air-flow obstruction is due to decreased inspiratory capacity.
Future work should evaluate the mechanisms that decrease inspiratory capacity across the severity of GLI-defined air-flow obstruction. Because inspiratory capacity is the volume that is maximally inspired from FRC, two mechanisms are considered. First, we have shown that FRC increased with more severe air-flow obstruction than with normal spirometry; thus, the inspiratory capacity may have been limited by the effect of hyperinflation on FRC. Second, prior work has shown that computed tomography-measured emphysema is strongly associated with GLI-defined severe air-flow obstruction18; thus, the inspiratory capacity may have been limited by compression of ventilated lung tissue by emphysematous regions. These mechanisms may also lead to a lower inspiratory capacity through reductions in respiratory muscle strength, a consequence of decreased curvature of the diaphragm (hyperinflation) or emphysema-associated sarcopenia (loss of diaphragmatic muscle mass and function).13,14,40,41 A decrease in inspiratory capacity is clinically meaningful, as it reduces the tidal volume response to exercise and, in turn, may lead to symptom-limiting dyspnea and exercise intolerance.30,31
Our results further demonstrate associations between the severity of GLI-defined air-flow obstruction and statistically significant lower values for diffusing capacity (single-breath diffusing capacity and hemoglobin-adjusted single-breath diffusing capacity). Because a decreased diffusing capacity describes impaired gas exchange, these results suggest that severe forms of air-flow obstruction in our study population were more likely due to COPD or the asthma-COPD overlap syndrome, rather than asthma alone (77.1% of our PFT sample population were former or current smokers).1
Our study has several strengths. We have evaluated a large study sample across a wide age range and applied a rigorous age-appropriate method for defining spirometric impairments. We have also avoided the limitations of reference equations for static lung volumes and diffusing capacity by using multivariable linear regression to calculate adjLSMeans and by expressing our results with height-cubed standardization.
We acknowledge, however, that a limitation of our study is the lack of a comparison with the more commonly used spirometric diagnostic algorithms from the Global Initiative for Obstructive Lung Disease (GOLD) and 2005 ATS/ERS task force.1,42,43 We posit that, in our study sample, such a comparison is seriously flawed for at least 3 age-related reasons. First, because aging impairs respiratory mechanics, the FEV1/FVC is often <0.70 in healthy never-smokers starting at about 45–50 y of age; GOLD establishes air-flow obstruction on the basis of FEV1/FVC < 0.70.8,9,13 Second, because aging is associated with greater variability in spirometric performance starting at about 40 y of age,8 diagnostic thresholds for FVC and FEV1 that are based on percent of predicted (measured/predicted × 100%) incorrectly assume a given value is equivalent for all persons, regardless of age, height, sex, and ethnicity; eg, GOLD uses FVC<80% of predicted to establish a restrictive pattern, and both GOLD and the 2005 ATS/ERS criteria use FEV1% of predicted to stage severity in air-flow obstruction.8,9,13,44 Third, the lower limit of normal for FVC and FEV1/FVC, as defined by the 2005 ATS/ERS task force, and the earlier described % of predicted thresholds for FVC and FEV1 are calculated from regression equations7 with an age range of up to 80 y; extrapolating beyond the age range of regression equations should be avoided (as per the ATS/ERS).1 Hence, in our study sample with a mean age of 64.6 y and an age range of up to 93 y, it is physiologically and clinically inappropriate to apply spirometric diagnostic algorithms that fail to rigorously account for age-related changes in lung function and/or include reference populations with a younger age range.13
We acknowledge four other study limitations. First, we did not determine whether changes in static lung volumes and diffusing capacity across spirometric impairments were associated with health outcomes, such as dyspnea (not available at the PFT visit). Second, our use of helium dilution rather than whole-body plethysmography may have limited the evaluation of TLC in air-flow obstruction.23,24 This may not be the case,45 however, because our results show that inspiratory capacity decreased while helium measured FRC increased across the severity of air-flow obstruction. Third, our study sample was limited to white and black races. Fourth, our results apply only to persons whose spirometric testing meets ATS acceptability criteria.1,22 In primary care settings and frail older persons, the ATS acceptability criteria for spirometric testing may be difficult to achieve, especially the end-of-test criterion.25,46,47
Finally, we consider that future work may establish appropriate reference equations for static lung volumes. If so, these reference equations may be used to determine if a high or low static lung volume is associated with health outcomes, independent of GLI-defined spirometric categories. In addition, because a low FVC (< lower limit of normal) in spirometric air-flow obstruction may be due to hyperinflation or restriction, the use of appropriate reference equations for static lung volumes may better establish the epidemiology of a mixed defect, defined by a low FEV1/FVC and low TLC (< lower limit of normal).1 These proposed analyses would further inform the role of static lung volumes in the evaluation of restrictive and obstructive physiology beyond what is already provided by GLI-calculated spirometric Z-scores.
Conclusions
Using GLI-calculated spirometric Z-scores and based on comparisons with normal spirometry, we found that a spirometric restrictive pattern is strongly associated with a restrictive ventilatory defect (decreased TLC, FRC, and RV), while spirometric air-flow obstruction is strongly associated with hyperinflation (increased FRC) and air trapping (increased RV and RV/TLC). Both spirometric impairments were also strongly associated with impaired gas exchange (decreased hemoglobin-adjusted single-breath diffusing capacity). Given these physiologic results and the previously established associations with multiple health outcomes,13–19,29 there is a strong rationale to implement GLI-based spirometric algorithms in clinical practice.18,48
Footnotes
- Correspondence: Carlos A Vaz Fragoso MD, 950 Campbell Ave, West Haven, CT 06516. E-mail: carlos.fragoso{at}yale.edu
The study was conducted at the VA Clinical Epidemiology Research Center (supported by a VA Merit Award [Dr Vaz Fragoso]) and the Yale Claude D Pepper Older Americans Independence Center (supported by P30AG021342).
All authors contributed to the analysis and interpretation of data and to the drafting the manuscript. Dr Vaz Fragoso and Mr Van Ness conceived and designed the study. Dr Vaz Fragoso and Ms Iannone collected the data. Dr Vaz Fragoso had full access to study data and takes responsibility for data integrity and accuracy of data analysis.
The authors have disclosed no conflicts of interest.
See the Related Editorial on Page 1228
- Copyright © 2017 by Daedalus Enterprises