Abstract
INTRODUCTION: It has been suggested that use of a high-flow nasal cannula (HFNC) could be a first-line therapy for patients with acute hypoxic respiratory failure. The purpose of this study was to determine if protocolized use of HFNC decreases unplanned intubation and adverse outcomes in an ICU population.
METHODS: The study was a prospective evaluation of 2 cohorts who received HFNC per protocol. Control groups were retrospective selections of subjects who received HFNC in the pre-protocol period. Cohort 1 (n = 88) received mechanical ventilation for ≥ 24 h and was extubated directly to HFNC following strict protocol criteria. Cohort 2 (n = 83) were placed on HFNC when oxygen requirements escalated (>4 L/min).
RESULTS: Cohort 1 did not differ from its control group in mortality, hospital stay, or ICU days, but there were significant decreases in incidence of Gram-negative pulmonary infection (30% vs 9%, P = .001) and use of bronchodilator therapy (81% vs 61%, P = .008). Failed extubation rates were nearly identical across groups, but time to re-intubation was shorter in the protocol group (24 vs 13 h, P = .19). Cohort 2 did not differ significantly from its control group in intubation rates or mortality, but subjects managed by protocol experienced significant decreases in ICU days (4 vs 3 d, P = .03) and hospital days (12 vs 8 d, P = .007). There was a trend toward fewer hours on HFNC (33 vs 24 h, P = .10) and faster time to intubation when HFNC failed (19 vs 9 h, P = .08).
CONCLUSIONS: Extubation to HFNC led to a significant decrease in pulmonary infections and bronchodilator therapy in Cohort 1 but did not reduce length of stay or rates of failed extubation. When HFNC was used early and per protocol (Cohort 2), ICU and hospital lengths of stay were reduced and HFNC was initiated more quickly when the need for respiratory support escalated.
- high-flow nasal cannula
- respiratory failure
- mechanical ventilation
- postextubation management
- re-intubation
- pulmonary infection
Introduction
High-flow nasal cannula (HFNC) has gained popularity due to its ease of use, comfort, and efficient delivery of humidified high-flow oxygen.1–4 It has been used successfully with medical ICU patients, as well as in postoperative cardiac, vascular, and trauma populations, to reduce work of breathing, improve accuracy of delivered FIO2, provide minor positive pressure delivery to airways, and wash out CO2.1,2,5 The literature suggests that early HFNC use may result in improved outcomes, but optimal patient characteristics, flows, FIO2 range, and timing are unclear.3 Gaunt et al6 found that subjects who received HFNC earlier rather than later in the hospital stay had reduced ICU and post-ICU lengths of stay, even after controlling for mechanical ventilation. It has been suggested that HFNC could be a first-line therapy for patients with acute hypoxic respiratory failure.6,7
HFNC has been used to resolve postextubation hypoxemia and to prevent re-intubation in certain patient populations because of its ability to reduce upper airway dryness after extubation, improve management of respiratory secretions, and potentially have a protective effect on mucociliary function.1,7–10 In addition, HFNC conditions the inspired gas, allowing for improved oxygenation, and alleviates tracheal mucosa inflammation after intubation.8,9 In subjects at low risk for re-intubation, Hernández et al11 demonstrated that, compared to conventional O2 therapy, only 1.5% of subjects extubated to HFNC required re-intubation for respiratory failure, compared to 9% in the control group.
There are, however, conflicting data regarding the benefits of HFNC postextubation.12,13 In a study of surgical subjects who received HFNC versus standard O2 therapy after postoperative extubation, there were no significant differences in rates of hypoxemia, need for O2 therapy after HFNC, pulmonary complications, or length of stay.13 Hernández et al14 investigated HFNC use in high-risk subjects, and re-intubation rates for respiratory failure were nearly identical (5%) for subjects receiving HFNC and noninvasive ventilation (NIV). Finally, a recent meta-analysis found no difference in rates of re-intubation for subjects extubated to HFNC compared to usual care.15
Some evidence supports the use of HFNC to prevent intubation in patients with an escalating need for respiratory support, but studies are limited by small sample sizes, retrospective design, and varying severity of respiratory distress. In a small prospective study of subjects requiring > 9 L/min of O2 to achieve SpO2 ≥ 92%, HFNC was associated with reduced breathing frequency, dyspnea, and pulse oximetry, but one quarter of subjects required invasive mechanical ventilation.16 In contrast, in subgroup analyses of a large multi-center trial, subjects with moderate to severe ARDS who were placed on HFNC had significantly lower intubation rates when compared with subjects who received standard O2 or NIV.17 It is unknown, however, what role HFNC should play when respiratory support requirements escalate. We found only one study that examined subjects as the need for respiratory support began to escalate. Parke et al18 found that only 10% of subjects who received HFNC for mild to moderate hypoxemic respiratory failure required intubation, compared to 30% in the control group (standard face mask).
Our study evaluated the prophylactic use of HFNC in 2 critically ill populations: newly extubated subjects and subjects with an escalating need for respiratory support. We evaluated whether protocols that standardize HFNC use in these populations prevented unanticipated respiratory compromise and adverse outcomes. Specifically, we hypothesized that receiving HFNC per protocol would reduce the rate of unplanned intubation or re-intubation in a heterogeneous ICU population.
QUICK LOOK
Current knowledge
High-flow nasal cannula (HFNC) has promise as a first-line therapy for critically ill patients with acute hypoxic respiratory failure. It has been shown to resolve postextubation hypoxemia and to prevent re-intubation in certain patient populations. In addition, there is evidence that HFNC reduces intubation rates if used prophylactically as the need for respiratory support escalates.
What this paper contributes to our knowledge
When HFNC was protocolized and used immediately after extubation, there was a significant decrease in pulmonary infection rates and bronchodilator therapy use, but the protocol did not reduce length of stay or prevent re-intubation. When the HFNC protocol was initiated proactively as the need for respiratory support escalated, ICU and hospital lengths of stay were reduced and respiratory distress was recognized more quickly.
Methods
Our study was a prospective evaluation of adults at Iowa Methodist Medical Center, UnityPoint Health, Des Moines, Iowa, which is a tertiary hospital with a mixed medical, surgical, and trauma ICU. HFNC was delivered via AIRVO (Fisher & Paykel, Auckland, New Zealand); at the time of the study, the modality was available only in the ICU. The devices were loaned to the study hospital by Fisher & Paykel; no funding was received from the manufacturer to conduct this study.
There were 2 populations in this study: Cohort 1 and Cohort 2. The investigation was a parallel, 2-group design. To determine sample sizes in both cohorts, power analyses were tested for the intervention to reduce intubation and re-intubation using the Gaunt et al6 study for comparison. To detect a moderate effect size with a power of 0.80,19 each cohort required a sample size of between 64 and 99 subjects. A subject could be in both cohorts only if HFNC was received as part of escalation of care (Cohort 2) and HFNC was administered after extubation (Cohort 1). The study was approved by the Institutional Review Board at the study hospital.
Procedures
Cohort 1: Extubation to HFNC.
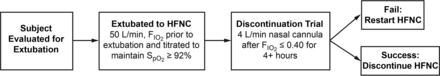
All subjects in Cohort 1 were admitted to the hospital during a 5-month study period (July–November 2015) and received mechanical ventilation in the ICU for 24 h or more. The study protocol is illustrated in Figure 1. Subjects were assessed for extubation readiness on a daily basis per hospital protocol (see the supplementary materials at http://www.rcjournal.com).20 After extubation, they were placed immediately on HFNC with initial flow at 50 L/min and FIO2 set to the last documented level and titrated to maintain SpO2 ≥ 92%. This 92% saturation threshold was chosen due to well-known physiologic behavior, which is outlined within the description of the oxyhemoglobin dissociation curve.21–23 According to this principle, as the curve becomes flattened at about 90% saturation, there is no relative value in the PaO2 increasing above 60 mm Hg. Conversely, as the hemoglobin becomes desaturated, the opposite is true. Creating a threshold of 92% gives the clinician some cushion should the patient deteriorate.
Study protocol for cohort 1.
When the FIO2 requirement was ≤ 40 for 4 consecutive hours, a discontinuation trial off HFNC was attempted using low-flow nasal cannula (4 L/min). If subjects were unable to maintain target saturations, they were restarted on HFNC at the last documented setting. If subjects maintained their saturations, HFNC was discontinued. During the study period, the protocol was used 104 times; however, 6 encounters were excluded because the subjects did not receive mechanical ventilation for > 24 h and 10 subjects received the protocol more than once. This resulted in 88 unique subjects in the study group for Cohort 1.
After the study period concluded, an equal number of subjects were identified retrospectively as a pre-protocol control group. Subjects in the control group were admitted prior to the study period (January–June 2015) and received mechanical ventilation for ≥ 24 h. Postextubation oxygenation modality was not guided by protocol but rather was selected at the discretion of the physician and respiratory therapist at the time of extubation. In the control group, subjects were most commonly extubated to nasal cannula, HFNC (Optiflow, Fisher & Paykel, Auckland, New Zealand), or bi-level positive airway pressure (BiPAP, Philips Respironics, Murrysville, Pennsylvania). One hundred forty-nine subject encounters were reviewed for study inclusion criteria; 61 (41%) were excluded from the control group because of tracheostomy (n = 9), failure to extubate or discharged from ICU with mechanical ventilation (n = 26), or terminal extubation (n = 26).
Cohort 2: Escalation to HFNC.
All Cohort 2 subjects were admitted to the hospital during a 7-month study period (July 2015 to January 2016) and demonstrated a need for increased respiratory support (>4 L nasal cannula or ≥ 36 FIO2 to keep SpO2 ≥ 92%). The study protocol is illustrated in Figure 2. Initial HFNC settings were 50 L/min, and FIO2 was titrated to maintain SpO2 ≥ 92%. When the FIO2 requirement was ≤ 40 for 4 consecutive hours, a trial off the HFNC was attempted using a low-flow nasal cannula (4 L/min). The protocol was used 98 times during the study period; however, 8 encounters were excluded from analyses because the subjects received mechanical ventilation prior to HFNC, 1 subject was excluded because of death within 24 h of hospital admission, and 6 were excluded because of protocol violations (receiving 0–3 L/min nasal cannula prior to HFNC). This resulted in 83 unique subjects in the study group for Cohort 2.
Study protocol for cohort 2.
After the study period concluded, an equal number of subjects were identified retrospectively as a pre-protocol control group. The control group was composed primarily of subjects who were analyzed in a previous study.6 The data were reviewed to ensure that all subjects received HFNC as part of escalation of care in the ICU when the O2 requirement was met (>4 L/min), and subjects were excluded if HFNC was administered after mechanical ventilation.
Study Variables
For both cohorts, demographic variables included age and sex. Subjects were considered do-not-resuscitate if they had a do-not-resuscitate order or a do-not-intubate order at any time during the hospital stay. Comorbidities were abstracted from the medical record after discharge, including history of cardiac disease, respiratory disease, and smoking (current or former). Bronchodilator therapy indicates whether the subject received ipratropium bromide and/or albuterol during the ICU stay.
The primary outcome of interest was unplanned intubation. In Cohort 1, we noted a failed extubation if a subject was reintubated for respiratory reasons within 72 h of extubation. For Cohort 2, we noted intubation after HFNC if a subject received mechanical ventilation within 72 h of HFNC initiation. Additional outcomes included mortality, lengths of stay in the hospital and in the ICU, and pulmonary infection. Pulmonary infection was obtained from culture data and indicated whether a subject had a Gram-negative pulmonary infection (eg, Acinetobacter, Klebsiella, Pseudomonas, Escherichia coli) while endotracheally intubated. At the study institution, cultures were obtained at the discretion of the attending physician when there was clinical suspicion of infection.
Statistical Procedures
All analyses were performed with IBM SPSS Basic Statistics for Windows, version 20.0 (IBM Armonk, New York). Descriptive statistics were examined and reported for continuous data as medians and interquartile ranges; categorical data were reported as counts and percentages. Statistical tests were 2-tailed and based on a 0.05 significance level. Because data were not normally distributed and sample sizes were unequal, differences between medians were assessed using the Kruskal-Wallis one-way analysis of variance. Differences between nominal variables were assessed using the chi-square test.
Results
Cohort 1. Extubation to HFNC
During the study period, 88 subjects met inclusion criteria for Cohort 1 and were managed by HFNC protocol; this was compared to a pre-protocol control group of 88 subjects. As shown in Table 1, the groups were similar in age, sex, acuity, and medical comorbidities. There were no statistically significant differences in ICU days, hospital days, or mortality.
Cohort 1: Extubation to HFNC
Extubation failure rates were similar across the pre-protocol and protocol phases (11% and 10%, respectively). For subjects who required re-intubation, the protocol group averaged more days between ICU admission and first extubation, as well as more days between first extubation and ICU discharge. Median time to re-intubation was reduced by 46% in the protocol group, but the difference was not statistically significant. In the protocol phase, there were significant decreases in incidence of Gram-negative pulmonary infection and use of bronchodilator therapy.
Cohort 2. Escalation to HFNC
Eighty-three subjects met inclusion criteria for Cohort 2; this was compared to a pre-protocol control group of 83 subjects. The groups were similar in demographics and comorbidities (Table 2). While intubation rates were similar across phases, subjects in the protocol group were intubated approximately 10 h earlier than subjects in the pre-protocol group (P = .08).
Cohort 2: Escalation to HFNC
Subjects managed without HFNC protocol had significantly more ICU days (4 vs 3 d, P = .03) and hospital days (12 vs 8 d, P = .007), and HFNC was initiated less quickly when the need for respiratory support escalated (1.4 h vs 0.3 h, P < .001). Mortality decreased from 28% in the pre-protocol group to 21% in the protocol group, but the difference was not statistically significant.
Discussion
HFNC has become a popular modality of respiratory support for critically ill patients, both as a postextubation therapy modality and as a preventive modality when patient respiratory status begins to deteriorate. Our study examined the utilization of HFNC for newly extubated subjects and those with escalating respiratory support requirements. Rates of unplanned intubation did not differ between the pre-protocol and protocol groups for either cohort. When subjects failed HFNC, however, time to intubation or re-intubation was approximately 10 h faster in the protocol groups, suggesting that HFNC protocols led to earlier recognition of respiratory failure and faster escalation of therapy. The protocol was efficacious in this mixed patient population, and results may be generalizable to broad, critically ill populations.
The effect of HFNC on length of stay was negligible in the postextubation cohort (Cohort 1), which is consistent with the literature,3,15 but hospital and ICU lengths of stay were shorter in the protocol phase for subjects with an increasing need for respiratory support (Cohort 2). This was an unexpected finding, as the introduction of a new protocol had the potential to lengthen the hospital stay by delaying discharge from ICU because HFNC is not available on the general in-patient floor at the study hospital. It is possible that HFNC may ultimately be useful for treating floor patients with mild to moderate hypoxemia, reducing unnecessary increases in level of care, but this hypothesis cannot be tested until HFNC therapy is available outside the ICU.
The pre-protocol and protocol groups in Cohort 1 averaged 4 d of mechanical ventilation prior to extubation. This is much longer than the average duration of mechanical ventilation in the Hernandez et al11 population (1–2 d) that was preselected to be at low-risk for extubation failure, as well as in other studies with populations that were extubated within 24 h of major surgery.12,13 The longer the duration of mechanical ventilation, the greater the risk of extubation failure. Our results might help shed new light on patients with moderate to severe disease who were excluded from earlier investigations.
The protocol group in Cohort 1 experienced a dramatic reduction in Gram-negative pulmonary infection. Other studies on postextubation pulmonary infection have focused on pneumonia13 or ventilator-associated pneumonia,7,11 but we selected Gram-negative infections as an outcome because they are associated with healthcare-acquired conditions that coexist with mechanical ventilation. During intubation, the artificial airway can provide a direct pathway for bacteria to colonize the lower airways and lead to lung infection. When compared to other O2 delivery devices, HFNC may be a superior modality because there is less mucociliary destruction and increased secretion clearance.1,15 Furthermore, intermediary devices, such as those that provide 6–15 L/min of supplemental O2, are unable to provide appropriate humidification. Normal respiratory physiology provides approximately 36–40 mg/L with an optimal required moisture of 44 mg/L (100% relative humidity at 37°C).24 Humidity levels below 25 mg/L for 1 h or 30 mg/L for 24 h or more are associated with airway mucosal dysfunction.25 In addition, previous evaluations have demonstrated that the unheated bubble humidifiers typically used in these intermediary devices perform no better than devices without humidification.26 This may explain in part why respiratory infection rates are lower when intermediary devices are bypassed and HFNC is used instead.
Bronchodilator use decreased dramatically in both cohorts, but the decrease was statistically significant in Cohort 1. This finding is consistent with our earlier work6,27 and with other literature.28 Prior to initiation of respiratory protocols at our hospital, respiratory therapy consultation was solicited at the discretion of the attending physician. We speculated that physicians and residents may have ordered nebulized bronchodilators inappropriately or as a proxy to obtain respiratory therapy consultation. The reduction in bronchodilator use suggests that care managed per protocol with respiratory therapy consultation may reduce unnecessary medication use, which lowers healthcare costs and reallocates vital resources. This finding deserves replication at other hospitals.
This study has several limitations. First, pre-protocol groups were selected retrospectively and it was difficult to ensure that they were similar in acuity to their respective protocol group. For example, Cohort 1 protocol subjects who failed extubation averaged 6 d between ICU admission and first extubation, compared to 4 d in the comparison group. This suggests that the protocol population may have had a higher severity of illness than the pre-protocol subjects. Second, we were unable to control for practice or provider differences that affected how cultures were collected in both the retrospective and protocol phases of the study. Cultures were collected using regular standard of care, and we did not set parameters or standards for this practice. Third, HFNC is not available on the general in-patient floor at the study hospital. As such, patients were excluded from the protocol in Cohort 2 if the need for respiratory support escalated on the floor without admission to the ICU. We acknowledge that this may have led to selection bias, favoring inclusion of subjects in Cohort 2 who had more severe respiratory disease. Fourth, the study sample is relatively small and subjects were derived from a mixed ICU population. Results cannot be applied to specific diagnoses or patient populations. Finally, we selected 4 L/min as the threshold for protocol initiation in Cohort 2 to allow for early identification of escalating respiratory support. The next step at the study hospital is to standardize this protocol in the ICU and investigate whether 4 L/min is the appropriate threshold for protocol initiation.
Conclusions
In an undifferentiated ICU population, the use of HFNC at early signs of hypoxemia and per protocol led to a significant decrease in respiratory infections, as well as trends toward shorter duration of HFNC therapy and less delay to definitive care when HFNC fails. These results indicate that there is a benefit to patients when standardized protocols for HFNC guide care decisions.
Acknowledgments
The authors thank Catherine Renner for her assistance in the study design, David Blake for his assistance with data collection, and Dustin McCann for helpful comments on the manuscript.
Footnotes
- Correspondence: Sarah K Spilman MA, Trauma Services, UnityPoint Health, 1200 Pleasant Street, Des Moines, IA 50309. E-mail: sarah.spilman{at}unitypoint.org.
Supplementary material related to this paper is available at http://www.rcjournal.com.
Mr Lamb presented an earlier version of this work at the annual conference of the Society of Critical Care Medicine, held January 21–25, 2017, in Honolulu, HI, and Dr Trump presented at the American Thoracic Society International Conference, held May 19–24, 2017, in Washington, DC.
Mr Lamb discloses relationships with Medtronic, and Fisher & Paykel. Dr Trump discloses a relationship with Fisher & Paykel. The other authors have disclosed no conflicts of interest.
See the Related Editorial on Page 367
- Copyright © 2018 by Daedalus Enterprises