Abstract
BACKGROUND: A ventilator-associated events (VAEs) algorithm was developed to detect events in mechanically ventilated subjects using objective parameters, and we aimed to use objective data of fluid balance to identify pulmonary edema-associated VAEs.
METHODS: This single-center retrospective cohort study was conducted in a medical ICU and enrolled all mechanically ventilated patients between July 2016 and June 2017. Electronic medical records were reviewed to obtain data regarding ventilator-associated conditions (VACs), infection-related ventilator-associated complications (IVACs), possible ventilator-associated pneumonia (VAP), and traditionally defined VAP.
RESULTS: Of the 1,158 mechanically ventilated subjects, 85 (7.3%) subjects developed VAEs with a corresponding incidence rate of 7.7 events per 1,000 ventilator days. Among the 85 subjects with VAEs, 52 (61.2%) were classified as IVACs, while 23 (27.1%) had possible VAP. Notably, pulmonary edema was the main etiology (29.0%) for VAEs in the 62 subjects with non-possible VAP VAEs. Compared with those without pulmonary edema, subjects with pulmonary edema had a higher positive fluid balance 2 d before (+1,228 vs +173.5 mL, P = .005) and 1 d before (+1,622 vs +313 mL, P = .002) the diagnosis of VAE. In the multivariate logistic regression analysis (adjusted odds ratio [OR]) adjusted for potential confounders, an older age (adjusted OR 1.072, 95% CI 1.001–1.147), receiving renal replacement therapy (adjusted OR 8.906, 95% CI 1.454–54.558), and a positive cumulative difference between fluid balance 2 d and 1 d before VAE indexing (adjusted OR 1.527 per L positive, 95% CI 1.153–2.023) were independently associated with pulmonary edema in subjects with VAEs.
CONCLUSION: These findings provide epidemiological evidence of VAEs in a medical ICU and showed that fluid balance may be used to identify pulmonary edema-associated VAEs. Further studies are warranted to validate and translate these findings into an automated surveillance system for VAEs.
- ventilator-associated events
- pulmonary edema
- fluid balance
- surveillance
- ventilator-associated conditions
- ventilator-associated pneumonia
Introduction
Ventilator-associated events (VAEs), using an objective and electronically computable surveillance algorithm, were introduced by the United States Centers for Disease Control and Prevention (CDC) to overcome the subjectivity of conventional diagnosis of ventilator-associated pneumonia (VAP). VAEs consist of ventilator-associated conditions (VACs), infection-related ventilator-associated complications (IVACs), and possible VAP.1 VAEs have been reported to be associated with an increased mortality rate, prolonged mechanical ventilation, and longer ICU length of stay.2–4 However, several studies have reported that traditionally defined VAP accounted for merely 25–40% of VAEs; therefore, the application of the VAE algorithm is somehow limited due to a relatively low positive predictive value for VAP resulting from a number of non-pneumonia VAEs, including pulmonary edema, atelectasis, and ARDS.5,6 Recent studies have found that 31–48% of VAEs were attributable to pulmonary edema.4,7,8 Given that data regarding fluid balance are routinely recorded in ICUs and should be automatically retrieved in most ICUs, we investigated whether objective fluid balance data might be used to identify pulmonary edema-associated VAEs. In this study, we used data over a 2-y period from a medical ICU and the VAE algorithm to investigate the incidence of VAEs, to analyze etiologies of non-possible VAP VAEs, and to use data regarding fluid balance for the identification of pulmonary edema-associated VAEs.
QUICK LOOK
Current knowledge
The ventilator-associated events (VAEs) algorithm was introduced to objectively detect events including ventilator-associated pneumonia (VAP) in mechanically ventilated subjects. VAEs consist of ventilator-associated conditions, infection-related ventilator-associated complications, and possible VAP. Pulmonary edema represents the majority of non-VAP VAEs and can be assessed objectively in most ICUs.
What this paper contributes to our knowledge
We provided evidence of the incidence and etiologies of VAEs in a medical ICU. A positive 2-d cumulative fluid balance before VAE indexing was associated with the development of pulmonary edema in subjects with VAE. Our findings imply that objective data regarding fluid balance may be used for early diagnosis of pulmonary edema-associated VAEs.
Methods
Study Design
This retrospective cohort study was conducted in a 24-bed medical ICU at a tertiary-care referral hospital with 1,514 beds in central Taiwan between July 2015 and June 2017. Electronic medical records were used to obtain relevant data on VAEs. The study was approved by the institutional review board of Taichung Veterans General Hospital (IRB number: CE18004B).
Measurements
In the study hospital, ventilatory parameters were interpreted by the respiratory therapist and electronically recorded at least twice a day. We reviewed electronic medical records to obtain ventilatory parameters and data regarding daily fluid records and other relevant characteristics including demographics, comorbidities, initial laboratory tests, and Acute Physiology and Chronic Health Evaluation (APACHE) II scores. Fluid status was represented as daily fluid balance prior to the date of diagnosis of VAE (ie, index date). Cumulative fluid balance prior to the development of VAEs was also evaluated for 2 d, 3 d, and 4 d prior to index.
Definitions of VAEs and VAP
The definition of VAEs was in accordance with the guideline proposed by the CDC.9,10 In brief, VAC is defined as at least 2 d of stable/decreasing ventilator settings followed by at least 2 d of FIO2 of at least 20% or an increase in PEEP of at least 3 cm H2O for at least 2 d. IVACs, a subset of VAC, is defined by an abnormal body temperature or white blood cell count and a new antimicrobial drug started and continued for >4 d. Possible VAP, a subset of IVACs, is defined by the presence of laboratory evidence of pneumonia, including a positive culture of lower respiratory secretions, a positive diagnostic test for specific respiratory viruses, or objectively defined purulent lower respiratory secretions. Traditional VAP was diagnosed based on the 2008 CDC criteria, which included the interpretation of chest radiographs.11 The etiologies for the VAEs were determined by the consensus of 2 chest physicians after reviewing serial chest radiographs, underlying diagnoses, laboratory findings, and the clinical/chest radiograph response to treatments including antibiotics or diuretics. Pulmonary edema was diagnosed based on the presence of infiltrate and effusion that was considered by a radiologist and 2 chest physicians to be consistent with pulmonary edema, lack of response to antibiotics, improved gas exchange or oxygenation with diuretics, and a negative fluid balance. Additionally, it is usual practice in all ICUs in Taiwan to have a high compliance with a bundle that includes elevation of head-of-bed, mouth care using chlorhexidine, daily assessment of sedation status, and spontaneous breathing trials.12
Statistical Analysis
Data were presented as frequencies (percentages) for categorical variables and as median (interquartile range) for continuous variables. Differences between 2 groups were analyzed using the Mann-Whiney U test for continuous variables and Fisher's exact test for categorical variables. Receiver operating characteristic analysis was utilized to determine the representative variable for dynamic fluid balance. A multivariate logistic regression model was constructed to identify independent variables that predicted pulmonary edema-associated VAEs in subjects with VAEs. Variables were considered as candidates for inclusion in the multivariate model if the associated univariate P value was < .20.13 Statistical significance was set at a 2-sided P value < .05. All data were analyzed using SPSS software version 22.0 (SPSS, Chicago, Illinois).
Results
Incidence of VAEs
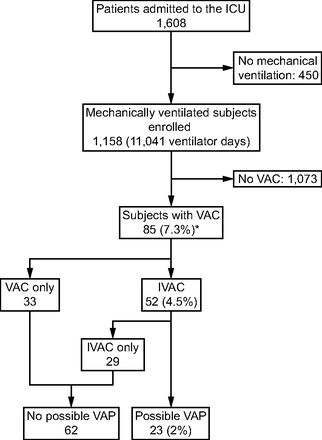
A total of 1,608 patients were admitted to the ICU between July 2015 and June 2017. Of these 1,608 patients, 450 patients without usage of mechanical ventilation were excluded; the remaining 1,158 mechanically ventilated subjects were eligible for estimation of VAEs. Of these 1,158 subjects, 85 (7.3%) subjects developed VAEs with a corresponding incidence rate of 7.7 events per 1,000 ventilator days. Among these 85 subjects with VAEs, 52 (61.2%) of them met the criteria for IVACs, and 23 (27.1%) of them fulfilled the criteria for possible VAP (Fig. 1). These data showed that 7.3% of mechanically ventilated subjects developed VAEs, and possible VAP accounted merely for 27.1% of VAEs.
Flow chart. VAC = ventilator-associated condition; IVAC = infection-related ventilator-associated complication; VAP = ventilator-associated pneumonia. * 7.7 events per 1,000 ventilator days.
Basic Characteristics of Subjects Having VAEs
Table 1 summarizes the demographic and other relevant data of subjects with VAEs, and we further categorized these subjects by possible VAP. The mean age was 66 (range, 53–79.5) y, and 81.2% were men. The most common underlying comorbidities were malignancy (37.6%) and type II diabetes mellitus (27.1%). Given that subjects with pulmonary edema-associated VAEs should be less likely to have a positive culture of lower respiratory secretions, which is the criteria for possible VAP, we hence categorized the 85 subjects into non-possible VAP and possible VAP groups. Notably, characteristics including mortality rate were similar between the non-possible VAP and possible VAP groups, indicating that non-possible VAP VAEs were critical events in addition to possible VAP, and that it is crucial to clarify etiologies among those with non-possible VAP VAEs.
Characteristics of 85 Subjects With VAEs Categorized by PVAP
Etiologies of Subjects Having VAEs
We next attempted to investigate underlying etiologies and the met criteria in the 85 subjects having VAEs. Of the 85 subjects with VAEs, traditionally defined VAP accounted for 25.9% of the VAEs, while pulmonary edema (22.4%), septic shock resulting from the extra-pulmonary origin (14.1%), and atelectasis (12.9%) were the main causes of non-VAP VAEs (Table 2). Notably, pulmonary edema (29.0%, 18 subjects) accounted for the majority of etiologies for VAEs in the 62 subjects with non-possible VAP VAEs. Additionally, we found that 15 of the 22 subjects having traditionally defined VAP were classified as possible VAP, whereas 5 subjects with VAP failed to meet the criteria for IVAC due to usage of antibiotics for < 4 d and 2 subjects failed to fulfill criteria of possible VAP due to > 10 squamous epithelial cells per low-power field in tracheal aspirate. The short duration of antibiotic use in these 5 subjects was attributed to mortality within 4 d after the development of VAE (Table 2). Moreover, given that criteria for VAEs included an increase in FIO2 or PEEP, we also investigated which criteria for VAEs were met among the 85 subjects with VAEs. Interestingly, each of these 2 criteria accounted for approximately 50% of the met criteria for VAC in both non-possible VAP and possible VAP groups. Taken together, these data clarified the etiologies of VAEs and showed that pulmonary edema accounted for the majority of non-possible VAP VAEs.
Etiologies, Met Criteria for VAE, and Time From Intubation to Event in Subjects Having VAEs Categorized by PVAP
Positive Fluid Balance Predicted Pulmonary Edema-Associated VAE
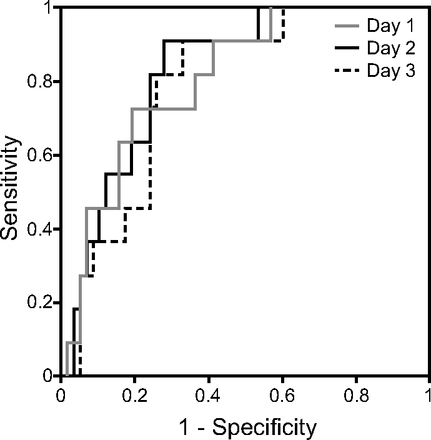
Given that objective data regarding fluid balance are available in most ICUs, we attempted to address whether dynamic fluid balance might be used to predict the development of pulmonary edema-associated VAEs. Table 3 details the daily and cumulative input (I), output (O), and fluid balance (I − O) data prior to the diagnosis-date of VAEs in the 85 subjects with VAEs categorized by pulmonary edema. Compared with those without pulmonary edema, subjects with pulmonary edema apparently had a higher positive fluid balance on 2 d (+1,228 vs +173.5 mL, P = .005) and 1 d (+1,622 vs +313 mL, P = .002) prior to the diagnosis date of VAE. We then used receiver operating characteristic analysis to determine the indicator for fluid status and found that the 2-d fluid balance had a better discriminative power than the 1-d or 3-d fluid balance to predict pulmonary edema among subjects having VAE but not receiving renal replacement therapy (Fig. 2). We next investigated which independent variables could predict pulmonary edema-associated VAEs. All of the variables were further categorized by pulmonary edema, and we set 0.20 as the level of beta error in univariate analysis (see the supplementary materials at http://www.rcjournal.com). For the individual data of the 19 subjects with pulmonary edema-associated VAEs, see the supplementary materials at http://www.rcjournal.com. Given that the APACHE II score is a composite severity score including white blood cell count and serum creatinine level, we used APACHE II scores to represent severity in the multivariate regression model.14 In the multivariate logistic regression model adjusted for gender, malignancy, hemoglobin, and APACHE II score, older age (adjusted odds ratio [OR] 1.072, 95% CI 1.001–1.147), receiving renal replacement therapy (adjusted OR 8.906, 95% CI 1.454–54.558), and a positive cumulative 2-d fluid balance (adjusted OR 1.527 per L increase, 95% CI 1.153–2.023) were independently associated with the diagnosis of pulmonary edema in subjects with VAEs (Table 4). Collectively, these data demonstrated the crucial role of fluid balance in pulmonary edema and found that a positive 2-d fluid balance was an independent predictor for pulmonary edema-associated VAE.
Daily Fluid Status of the 85 Subjects With VAEs Categorized by Pulmonary Edema
Performance of fluid balance with different time-window to predict pulmonary edema in subjects having VAC without hemodialysis. Receiver operating characteristic curves showing the performance of fluid balance 1 d before VAE indexing (AUC 0.809, 95% CI 0.687–0.931), fluid balance 2 d before indexing (AUC 0.828, 95% CI 0.718–0.937), and fluid balance 3 d before indexing (AUC 0.787, 95% CI 0.665–0.909) in predicting pulmonary edema among subjects with VAC without hemodialysis. VAC = ventilator-associated complications; AUC = area under the curve.
Logistical Regression to Predict Pulmonary Edema in Subjects With VAEs
Discussion
In this study, we investigated the incidence rate of VAEs in a medical ICU and the role of fluid balance in predicting pulmonary edema-associated VAEs. We found that 7.3% of subjects developed VAEs with a corresponding incidence rate of 7.7 events per 1,000 ventilator days, and pulmonary edema was the main etiology of non-possible VAP VAEs. A positive 2-d cumulative fluid balance was independently associated with the development of pulmonary edema-associated VAEs. These findings provide epidemiological evidence of VAEs in mechanically ventilated subjects and imply the potential of incorporating objective data regarding fluid balance into automated surveillance for VAEs.
A number of studies have characterized the incidence rate of VAE, ranging from 5% to 10% in mechanically ventilated subjects or from 2 to 12 events per 1,000 ventilator days.2,7,8,10 Rawat et al15 conducted a longitudinal quasi-experimental study that included 38 hospitals in Maryland and Pennsylvania between 2012 and 2015 and reported that the incidence rate was approximately 5 events per 1,000 ventilator days after the implementation of bundle care consisting of head-of-bed elevation, use of subglottic secretion drainage endotracheal tubes, oral care, chlorhexidine mouth care, and daily spontaneous awakening and breathing trials. In our ICU, bundled care with head-of-bed elevation, oral mouth care using chlorhexidine, daily assessment of sedation status, and spontaneous breathing trials was implemented for all participants and checked daily during the study period, and the incidence of VAE was 7.7 events per 1,000 ventilator days, which is similar to the data reported by Rawat et al15 (Fig. 1). In addition, the etiologies of VAEs in this study were consistent with previous studies that traditionally defined VAP, pulmonary edema, atelectasis, and acute respiratory distress syndrome were main etiologies of VAEs (Table 2).16
A positive fluid balance has been implicated in the development VAEs. Lewis et al,17 in a case-control study involving 110 subjects with VAC and 110 subjects without VAC, reported that a positive fluid balance was associated with a risk of VAC (OR 1.2 per L increase, 95% CI 1.0–1.4). Nakahashi et al18 also found that the development of edema was positively associated with the occurrence of VAC in a retrospective cohort study enrolling 303 mechanically ventilated subjects (adjusted hazard ratio 2.145, P = .037). Additionally, in line with our data, Kobayashi et al19 reported that receiving renal replacement therapy was an independent risk factor for IVACs and VAC, and is reflected in our data, which showed that receiving renal replacement therapy was associated with pulmonary edema-associated VAEs (Table 4). Collectively, this evidence suggests that a positive fluid balance was associated with the development of VAEs, particularly pulmonary edema-associated VAEs.
Currently, a limited overlap between possible VAP and traditionally defined VAP remains an essential issue in the application of the VAE algorithm, although the VAE algorithm aims to broaden the scope of surveillance in addition to surveying traditionally defined VAP alone.8,20,21 Indeed, patients with traditionally defined VAP may not meet the criteria for possible VAP given that the requirement of the use of antibiotics for > 4 d and the presence of laboratory evidence of pneumonia may exclude a number of subjects who have traditionally defined VAP. In this study, 7 of the 22 subjects with traditionally defined VAP failed to meet criteria for IVACs because the duration of antibiotic use was < 4 d in 5 subjects, and the other 2 subjects failed to meet criteria of possible VAP due to the lack of laboratory evidence of pneumonia in tracheal aspirate (Table 2).
Automated surveillance of ventilator-associated complications is the goal of the VAE algorithm, and interpretation of the chest radiograph was thus excluded in the VAE algorithm due to the need for subjective interpretation.22 Interestingly, the lack of radiograph interpretation has been, at least partly, a potential limitation of VAE,23,24 and we thought that interpretation of serial chest radiographs should be helpful to determine etiologies, particularly in pulmonary edema, atelectasis, and ARDS, for VAEs as shown in this study. Notably, the VAE algorithm is designed not only to overcome the subjectivity of traditional VAP definitions but also to broaden the focus of surveillance beyond pneumonia alone and to enable automated surveillance of events in mechanically ventilated subjects.25,26 We thus thought that the framework of the VAE algorithm could potentially be further optimized through incorporating objective parameters identified through analyzing the VAE etiology to achieve automated surveillance of VAEs and providing the likely etiology.
Furthermore, instead of merely being a tool for the surveillance of VAEs, the long-term goal of the VAE algorithm should be to improve outcomes of mechanically ventilated subjects through prevention of VAEs.5 As shown by Rawat et al,15 the incidence of VAEs was reduced by 37.6% after implementation of a multifaceted intervention, highlighting the substantial role of the VAE algorithm in improving patient outcomes. Accumulating evidence have shown the crucial need of the conservative fluid strategy in critically ill patients,27 and we identified in other work the critical role of cumulative fluid balance in critically ill influenza subjects.28 Therefore, the findings of this study suggest that a positive cumulative fluid balance may be a real-time indicator for VAE prevention by providing a warning that a patient is on track to develop a VAE secondary to pulmonary edema.
This study has limitations. First, this was a retrospective study in a single ICU, although the incidence and etiologies of VAEs in this study were similar to recently published prospective multi-center studies.10,15 Second, the generalizability of our findings to ICUs other than medical ICUs could be limited given that the studied ICU is a respiratory ICU. Third, we did not survey traditionally defined VAP and fluid balance in those without VAEs given that our main focus was to identify pulmonary edema-associated VAEs. Fourth, the standard for pulmonary edema was clinical judgment, which is by nature a subjective evaluation.
In conclusion, we found that the incidence of VAEs was 7.7 events per 1,000 ventilator days in a medical ICU, and objective data regarding fluid status may be used to predict pulmonary edema-associated VAEs. These findings may help in the development of automated surveillance for VAEs in the future. In addition, given that the long-term goal of surveillance for VAEs is to prevent the occurrence of VAEs, instead of simply to monitor for VAEs, our findings merit further study to validate that fluid status might be used as an early warning sign of VAEs resulting from pulmonary edema.
Footnotes
- Correspondence: Ming-Cheng Chan MD PhD, Division of Respiratory Therapy, Department of Internal Medicine, Taichung Veterans General Hospital, 1650 Taiwan Boulevard Sect. 4, Taichung, Taiwan 40705, Republic of China. E-mail: mingcheng.chan{at}gmail.com.
The authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
- Copyright © 2018 by Daedalus Enterprises