Abstract
BACKGROUND: Most children are exposed to human metapneumovirus (HMPV) by the age of 5 y. This study aimed to describe the morbidity associated with HMPV infections in a cohort of children in the Midwest of the United States.
METHODS: This was a retrospective 2-center cohort study including children (0–17 y old) hospitalized with HMPV infections at 2 tertiary care pediatric hospitals from 2009 to 2013. Demographics, chronic medical conditions, viral coinfections, and hospitalization characteristics, including the need for respiratory support, high-flow nasal cannula, CPAP, bi-level positive airway pressure, invasive mechanical ventilation, pediatric ICU admission, acute kidney injury (AKI), use of extracorporeal membrane oxygenation, and length of stay, were collected.
RESULTS: In total, 131 subjects were included. Those with one or more comorbidities were older than their otherwise healthy counterparts, with a median age of 2.8 y (interquartile range [IQR] 1.1–7.0) compared to 1.3 y (IQR 0.6–2.0, P < .001), respectively. Ninety-nine (75.6%) subjects required respiratory support; 72 (55.0%) subjects required nasal cannula, simple face mask, or tracheostomy mask as their maximum support. Additionally, 1 (0.8%) subject required high-flow nasal cannula, 1 (0.8%) subject required CPAP, 2 (1.5%) subjects required bi-level positive airway pressure, 15 (11.5%) subjects required invasive mechanical ventilation, 4 (3.1%) subjects required high-frequency oscillatory or jet ventilation, and 4 (3.1%) subjects required extracorporeal membrane oxygenation. Fifty-one (38.9%) subjects required pediatric ICU admission, and 16 (12.2%) subjects developed AKI. Subjects with AKI were significantly older than those without AKI at 5.4 y old (IQR 1.6–11.7) versus 1.9 y old (IQR 0.7–3.5, P = .003). After controlling for the presence of at least one comorbidity and cystic fibrosis, each year increase in age led to a 16% increase in the odds of AKI (P = .01). The median length of stay for the entire cohort was 4.0 d (IQR 2.7–7.0).
CONCLUSIONS: Children hospitalized with HMPV may be at risk for AKI. Risk of HMPV-associated AKI appears to increase with age regardless of severity of respiratory illness or presence of comorbidities.
- human metapneumovirus
- extracorporeal membrane oxygenation
- acute kidney injury
- hospitalized
- pediatric intensive care unit
- respiratory failure
Introduction
Serologic evidence of exposure to human metapneumovirus (HMPV) by the age of 5 y is present in most children.1 HMPV is a member of the paramyxovirus family, similar to respiratory syncytial virus (RSV).2 HMPV has been recognized as a significant cause of morbidity, primarily in children presenting with acute respiratory illness, although elderly or immunocompromised adults are also at risk of severe disease.3,4 Children hospitalized with HMPV infection are, on average, older than children hospitalized with RSV infection, although the majority of cases occur in children < 2 y old.3,5,6
Clinical symptoms of HMPV infection are similar to those seen in RSV infection, including fever, tachypnea, cough, rhinorrhea, retractions, and wheezing.7 Most HMPV infections occur during late winter and spring.8,9 Significant variation has been noted in the number of HMPV cases each year as well as in the proportion of children with HMPV who required hospitalization versus out-patient monitoring.10 One prospective study estimated the prevalence in children under 14 y of age hospitalized for acute respiratory illness at 5.5%.11 A separate study in the in-patient, out-patient, and emergency department settings estimated the prevalence at 6–7% in children < 5 y of age presenting with acute respiratory illness or fever.8
Because the use of multiplex polymerase chain reaction technology to detect numerous respiratory pathogens has become more widely available at our institutions, we have observed a significant number of children admitted with critical illness in the setting of HMPV infection. The spectrum of illness includes severe respiratory failure requiring extracorporeal membrane oxygenation (ECMO). Additionally, common respiratory viruses such as HMPV have been associated with distant end organ damage such as acute kidney injury (AKI). The goal of this observational study was to describe the morbidity associated with HMPV infections in a cohort of children in the Midwest of the United States.
QUICK LOOK
Current knowledge
Most children are exposed to human metapneumovirus (HMPV) by the age of 5 y, and HMPV has been recognized as a significant cause of morbidity in children and adults. This includes a spectrum of illness that ranges from mild upper respiratory symptoms to severe respiratory failure.
What this paper contributes to our knowledge
We evaluated pediatric subjects hospitalized with HMPV-related illness via a retrospective chart review at 2 children's hospitals in the U.S. Midwest. The overall need for respiratory support was higher in subjects with any comorbidities compared to otherwise healthy children, as well as specifically higher in those with a history of asthma or prematurity. The risk of acute kidney injury increased with age regardless of comorbidities or severity of respiratory illness. Severe respiratory failure requiring extracorporeal membrane oxygenation support occurred in subjects with bacterial or fungal coinfection.
Methods
The Institutional Review Boards at the University of Michigan and Wayne State University approved this study with waiver of parental permission. A retrospective medical record review was performed for all subjects aged 0–17 y admitted to the University of Michigan Health System and Children's Hospital of Michigan with laboratory-confirmed HMPV infection between January 2009 and May 2013. Both institutions have large multidisciplinary pediatric ICUs, which included 26 beds at University of Michigan and 32 beds at Children's Hospital of Michigan during the study period. Detection of HMPV infection occurred with polymerase chain reaction or viral culture on specimens obtained via throat or nasopharyngeal swab or via bronchoalveolar lavage. Neonates who were hospitalized since birth and patients admitted for scheduled surgery were excluded. If a subject was admitted more than once with HMPV during the study period, only the index hospitalization was included.
Demographic data collected included gender, age, and race (ie, white, African-American, Asian, other). Chronic medical conditions were identified via International Classification of Diseases, Ninth Revision (ICD-9) codes, including cystic fibrosis, asthma, bronchiectasis, pulmonary hypertension, congenital heart disease, congestive heart failure, neutropenia, and prematurity. Viral coinfection was defined as positive testing for RSV or influenza A or B by polymerase chain reaction from the same sources as described above.
Hospitalization characteristics included the need for supplemental oxygen, high-flow nasal cannula, CPAP, bi-level positive airway pressure, invasive mechanical ventilation, pediatric ICU admission (including both the pediatric ICU and pediatric cardiothoracic unit), development of AKI (per ICD-9 coding), use of ECMO support, pediatric ICU and hospital length of stay, and month of admission. For subjects who did not require respiratory support, we collected the indication for hospital admission.
Where available, we collected baseline and peak serum creatinine, as well as subject height, to determine the absolute and relative change in creatinine and the estimated glomerular filtration rate using the modified Schwartz equation.12 The estimated glomerular filtration rate was only determined for children ≥ 1 y of age or older because the modified Schwartz equation is not valid for children < 1 y old. Urine output was not available.
Demographics, comorbid conditions, and coinfections were analyzed for associations with hospitalization characteristics using chi-square tests or Fisher exact tests (ie, when expected number in cell was <5) for categorical variables and non-parametric Mann-Whitney U tests for continuous, non-normally distributed dependent variables (ie, pediatric ICU and hospital length of stay). AKI per ICD-9 coding and need for supplemental oxygen were additionally analyzed for predictive value in relation to the remaining hospitalization characteristics. Non-parametric Mann-Whitney U tests and logistic regressions were also used to test whether there was an association between age and the presence of one or more comorbid conditions as well as each of the hospitalization characteristics listed above. If multiple independent variables were found to be associated with one of the binary dependent variables in the above analyses, a multivariable logistic regression was performed with each of the associated independent variables as well as any covariates associated with the independent variables to determine the best predictors of the dependent variable. Alpha was set at 0.05 for significance. Data were analyzed with SPSS 25 (IBM, Armonk, New York).
Results
Subject Characterization
A total of 138 cases of HMPV confirmed with polymerase chain reaction or viral culture were identified at the 2 institutions during the study period. Seven cases were excluded. Two of the excluded cases were hospitalized since birth in the neonatal ICU with respiratory failure secondary to prematurity. Two other excluded cases were admitted for planned cardiac operations, and one was admitted for planned tracheostomy placement; each tested positive for HMPV after the procedure. Finally, two patients were admitted twice with HMPV during the study period; for these subjects, only the index hospitalization was included in the analysis. After exclusions, a total of 131 subjects were included.
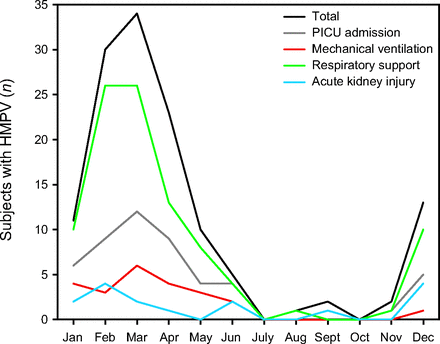
Demographics, comorbidities, and viral coinfections are summarized in Table 1. Eight-five (64.9%) subjects were male. Median age at admission was 2.0 y (interquartile range [IQR] 0.8–3.9). Eighty-six (65.6%) subjects were white, 27 (20.6%) were African-American, and 2 (1.5%) were Asian. At least one of the surveyed comorbidities was present in 81 (61.8%) subjects. Asthma was the most common comorbidity, noted in 56 (42.7%) subjects, followed by prematurity in 30 (22.9%) subjects, congenital heart disease in 15 (11.5%) subjects, and cystic fibrosis in 12 (9.2%) subjects. Overall, subjects with one or more comorbidities were more likely to be older, with a median age of 2.8 y (IQR 1.1–7.0), than their otherwise healthy counterparts, with a median age of 1.3 y (IQR 0.6–2.0, P < .001). By logistic regression, with each year increase in age, there was a 13% increase in the odds of having at least one comorbidity (P = .02). Viral coinfection was present in 15 (11.5%) subjects, with influenza in 9 (6.9%) and RSV in 6 (4.6%). HMPV-related hospitalizations peaked between February and March, with 85% of cases occurring between December 1 and April 30 (Fig. 1).
Subjects diagnosed with HMPV per month across the study period (n = 131). Additionally, number of subjects requiring respiratory support, PICU admission, mechanical ventilation, and diagnosed with acute kidney injury. Cases peaked between February and April. HMPV = human metapneumovirus; PICU = pediatric ICU.
Demographics, Comorbidities, and Viral Coinfection in Children With Human Metapneumovirus Infection
Hospitalization Characteristics
Hospitalization characteristics are summarized in Table 2. A total of 99 (75.6%) children required some form of respiratory support; 72 (55.0%) required nasal cannula, simple face mask, or tracheostomy mask as their maximum support. Additionally, 1 (0.8%) subject required high-flow nasal cannula, 1 (0.8%) subject required CPAP, 2 (1.5%) subjects required bi-level positive airway pressure, 15 (11.5%) subjects required invasive mechanical ventilation via endotracheal intubation or via preexisting tracheostomy, 4 (3.1%) subjects required high-frequency oscillatory or jet ventilation, and 4 (3.1%) subjects required ECMO as their maximum support. Of the 15 subjects treated with invasive ventilation, 10 received pressure control intermittent mandatory ventilation, 2 received pressure-regulated volume control, and 1 subject received pressure control continuous mandatory ventilation; for 2 subjects, the mode could not be determined from the medical record. Of the 32 (24.4%) subjects who did not require respiratory support, indications for hospital admission included fever complicating malignancy or sickle cell disease (n = 9), secretion management (n = 8), pneumonia not responding to out-patient antibiotic therapy (n = 4), sepsis (n = 3), fever (n = 2), cystic fibrosis exacerbation (n = 2), asthma (n = 2), and other non-respiratory bacterial infection (n = 2). Pediatric ICU admission was necessary for 51 (38.9%) subjects, with a median pediatric ICU length of stay of 2 days (IQR 1.0–11.0).
Hospitalization Characteristics of Children With Human Metapneumovirus Infection
Sixteen (12.2%) children were diagnosed with AKI. Among children with AKI, one had missing baseline creatinine value. For the remaining 15 children diagnosed with AKI, serum creatinine increased 0.4 ± 0.5 mg/dL representing a relative increase of 170% ± 150% from baseline. In terms of the estimated glomerular filtration rate, 14 of 15 children were one year of age or older. The estimated glomerular filtration rate declined by 70 ± 72 mL/min/1.73 m2 representing a relative decrease of 41% ± 28% from baseline.
Among the 115 subjects who were not diagnosed with AKI by their providers, 84 (73%) had their creatinine checked at least once during the hospitalization, and 31 (27%) had baseline creatinine data available. Average creatinine change from baseline was 0.1 ± 0.1 mg/dL, which represented an increase of 27 ± 40%. For the 12 (10%) subjects who were ≥ 1 y old, had a peak and baseline creatinine value, and had a recorded height, the average absolute estimated glomerular filtration rate decrease was 23 ± 52 mL/min/1.73 m2, or an average 14 ± 23% decrease from baseline.
Four (3.1%) subjects required ECMO support. One subject, with a course complicated by vancomycin-resistant enterococcus bacteremia, died during hospitalization. The median length of hospital stay for all subjects was 4.0 d (IQR 2.7–7.0).
Associations between age and hospitalization characteristics are summarized in Table 3. Subjects diagnosed with AKI were significantly older, with a median age of 5.4 y (IQR 1.6–11.7), than subjects who were not diagnosed with AKI, who had a median age of 1.9 y (IQR 0.7–3.5, P = .003). Otherwise, there were no significant age differences based on need for respiratory support, pediatric ICU admission, invasive ventilation, ECMO support, or length of stay (P > .05).
Relationships Between Age and Hospitalization Characteristics
Subjects with at least one of the measured underlying comorbidities had an increased frequency of requiring respiratory support compared to previously healthy subjects (odds ratio [OR] 2.26, 95% CI 1.01–5.10, P = .045). Increased need for respiratory support was also noted specifically in subjects with a history of asthma (OR 2.82, 95% CI 1.16–6.89, P = .02) or prematurity (OR 3.63, 95% CI 1.02–12.90, P = .036). Those admitted for neutropenia were at decreased risk of requiring respiratory support (P = .005; we were unable to determine an odds ratio because none of the 3 subjects with neutropenia received respiratory support). There were no other significant associations between any of the subjects' demographics or comorbidities in regard to need for respiratory support.
Subjects with cystic fibrosis had a significant increased risk of AKI (OR 4.46, 95% CI 1.17–17.03, P = .041). Need for respiratory support was significantly associated with an increased risk of need for ICU-level care (OR 9.1, 95% CI 2.60–31.83, P < .001) and a longer median length of stay, ie, 5 d (IQR 3–10) versus 3 d (IQR 1–4, P < .001).
Otherwise neither sex, viral coinfection, individual surveyed comorbidities, nor the general presence of one or more comorbidities were associated with increased risk of need for pediatric ICU admission, mechanical ventilation, ECMO support, AKI, or longer length of stay (P > .05). AKI was not increased in subjects with other markers of disease severity, including need for respiratory support, pediatric ICU admission, or mechanical ventilation, nor was it associated with an increased length of stay (P > .05).
On the basis of these findings, we performed a multivariable logistic regression including age, the presence of one or more comorbidities, and cystic fibrosis specifically. The results indicated that age was an independent predictor of risk of AKI (Table 4). The presence of one or more comorbidities was included due to its strong co-variation with age. After controlling for the presence of one or more comorbidities and cystic fibrosis, each year increase in age led to a 16% increase in odds of being diagnosed with an AKI (P = .01).
Multivariable Logistic Regression Analysis of Age, Cystic Fibrosis, and Presence of One or More Comorbid Conditions as Predictor for Acute Kidney Injury
Table 5 summarizes the clinical data for the 4 subjects who received ECMO. All 4 subjects had underlying lung disorders and at least one blood or sputum culture positive for bacteria or fungi. All 4 subjects received venovenous ECMO and survived to discharge.
Case Summaries of Subjects Requiring ECMO Support
Discussion
To our knowledge the risk of AKI in our cohort of subjects with HMPV infection appeared to increase with age. Although older children were also more likely to have at least one comorbidity, the presence of specific comorbidities, aside from cystic fibrosis, was not associated with AKI. A recent large study using a national hospitalization database of > 2.5 million children in the United States reported the incidence of AKI to be 0.39%; the risk of AKI increased with age, with the median age of subjects with AKI being 10.8 y versus 2.0 y in subjects without AKI.13 Given that the incidence of AKI was 12.2% in our study, it appears that children with HMPV may be at higher risk of AKI than the general hospitalized pediatric population. A separate study of a general pediatric ICU population demonstrated an incidence of AKI as high as 26.9%,14 whereas the rate among children admitted to the pediatric ICU was 13.7% in our study. The development of AKI was not associated with the need for respiratory support, mechanical ventilation, or pediatric ICU admission in our study. This suggests that AKI during HMPV infection may not be strictly related to hypoxemia or hemodynamic instability, and that HMPV may have a more direct effect on the kidneys.
Other respiratory viruses have been associated with AKI via various mechanisms. Rat models have demonstrated direct viral infection of kidney tissues as well as cytokine-mediated kidney injury via infection with RSV.15,16 Rhabdomyolysis-induced kidney injury is most commonly associated with influenza as well as other causes, including coxsackie virus, human immunodeficiency virus, and parainfluenza.17,18
The presence of AKI in our cohort was based on ICD-9 coding; thus, we were unable to evaluate for the possible cause or classification of AKI. The mean degree of change in serum creatinine and estimated glomerular filtration rate among our subjects with AKI is consistent with AKI defined by either the KIDGO group (creatinine increase of 0.3 mg/dL or 150–200% of baseline, or urine output < 0.5 mL/kg/h for 6 h) or the modified RIFLE criteria (decrease in estimated glomerular filtration rate of 25% or urine output < 0.5 mL/kg/h for 8 h).19,20 Urine output could not be determined accurately in our retrospective study. No data were collected regarding urinalysis results or creatinine phosphokinase levels in our cohort, so we are unable to comment further on the role of rhabdomyolysis. Future studies that evaluate a larger subset of HMPV-infected children with AKI would be useful to confirm our findings and delineate this risk further.
Although the incidence of HMPV decreases with age,21 it appears that the presence of comorbid conditions may be a risk factor for HMPV-related illness requiring hospitalization in older children. Comorbidities occurred at a higher rate among older children in our cohort. These findings are similar to a previous report in a general hospitalized population with HMPV showing 83% prevalence of comorbidities in subjects 5–17 y old versus 36% in those < 6 months old.10 Similar findings have been reported by others.22 Our cohort's baseline comorbidity rate (62%) was also comparable to those reported in other studies, which ranged from 56% to 68%.6,10,23 Children hospitalized with HMPV are also more likely to have underlying comorbidities than children with RSV in a combined out-patient and in-patient cohort.8 Our findings imply that, although HMPV typically affects younger children, with increasing age it may be those with comorbidities who are at risk of requiring hospitalization.
Previous studies have reported mixed results on specific risk factors that contribute to worse hospital outcomes due to HMPV. In a pediatric ICU cohort, increased mortality was observed in females, those with underlying medical conditions, and those with hospital-acquired HMPV. However, a difference in hospital or pediatric ICU length of stay or need for mechanical ventilation based on demographics or comorbidities was not found.22 Comparatively, children with comorbidities with HMPV have been reported to have a significant increase in pediatric ICU admission (OR 4.3) and intubation (OR 7.1).10 These children also had an overall longer stay and more expensive hospital care. In that cohort, length of hospital stay was specifically greater in subjects with non-asthma pulmonary disease, neuromuscular diseases, and metabolic diseases compared to subjects with asthma or cardiac issues.10 Increased length of stay, need for ICU-level care, and longer use of noninvasive ventilation and supplemental oxygen in pediatric subjects with HMPV and underlying comorbidities have also been reported.23
In comparison, our study identified the general presence of one or more of the specified underlying comorbidities to be a risk factor for needing respiratory support compared to previously healthy subjects. A history of asthma or prematurity conferred increased risk of respiratory support need. Subjects who were admitted for neutropenia in the setting of HMPV infection were at a lower risk of needing respiratory support, likely due to the indication for admission being a febrile neutropenia workup. This was also noted in previous studies.23
Outside of these associations and the discussed AKI data, our results did not indicate any other underlying risk factors with regard to increased length of stay, ICU admission, or mechanical ventilation. It is important to note that some of the previous studies included central nervous system diseases, genetic syndromes, seizures, neuromuscular diseases, and chronic renal disease in their comorbidities, which may have an important impact on HMPV outcomes.
In our study cohort, 4 subjects with severe respiratory failure required ECMO. All 4 of these subjects had underlying lung disease and developed ARDS. All of their hospital courses were complicated by either bacterial or fungal coinfection. Although the children in our cohort who required ECMO survived to discharge, they had prolonged hospital stays. A previous report described 2 of 111 pediatric subjects admitted to 1 of 3 pediatric ICUs with laboratory-confirmed HMPV who required ECMO.22 Several case reports have described the need for ECMO in pediatric subjects with HMPV, including a 32-month-old patient with asthma who initially failed high-frequency oscillatory ventilation,24 a 3-month-old infant who had been born at 27 weeks gestational age,25 and a 9-month-old subject with biliary atresia who developed HMPV-related respiratory failure in the days following liver transplant.26 All of these patients survived. Two pediatric patients a 7-month-old infant with acute lymphoblastic leukemia27 and a previously healthy 2-year-old child who developed bilateral hemorrhagic bronchopneumonia, died despite the use of ECMO.28
Our study has several limitations, including its retrospective nature, the use of ICD-9 codes to identify comorbidities, and the lack of inclusion of some comorbidities, such as neurologic and genetic conditions, that potentially are associated with hospital outcomes in other studies. The use of ICD-9 codes to identify AKI is also a limitation because this makes it difficult to draw definitive conclusions about the incidence of AKI during HMPV infection. The lack of urine output data as a supporting criterion for AKI diagnosis is another limitation. Additionally, as a 2-center study, we lacked sufficient numbers of pediatric subjects to provide a well-powered analysis of the multiple potential factors that could be associated with rare outcomes, including the development of AKI, need for ECMO, and death. Finally, not all subjects were tested for HMPV upon admission during the study period, and some cases were likely missed. Testing for HMPV was done at the discretion of the clinical care teams because no protocol or algorithm was in place for testing for HMPV.
Conclusions
Our results indicate that children hospitalized with HMPV may be at risk for AKI. Risk of HMPV-associated AKI appears to increase with age, regardless of severity of respiratory illness or presence of comorbidities. The mechanism of such kidney injury and any predisposing risk factors should be a focus of future study. Screening and treatment of AKI should be included during the in-hospital management of older children with HMPV infection.
Acknowledgments
We thank Dr Jenalee R Doom, University of Michigan Department of Pediatrics, for assistance with statistical analyses.
Footnotes
- Correspondence: Michael W Quasney MD PhD, The University of Michigan, 1500 E. Medical Center Drive, F6790 UH South, Box 5243, Ann Arbor, MI 48109. E-mail: mquasney{at}med.umich.edu
Dr Ashbrook presented a version of this paper at the Wayne State University Medical Student Research Symposium, held January 14-15, 2016, in Detroit, Michigan.
The authors have disclosed no conflicts of interest.
- Copyright © 2020 by Daedalus Enterprises