Abstract
BACKGROUND: Because standard home oxygen (O2) systems deliver O2 at fixed rates, these systems are not designed to ensure optimal oxygen delivery based on physiologic need. We tested the ability of the AccuO2 (OptiSat Medical, Minneapolis, Minnesota), a portable, closed-loop, oximetry-driven, O2-conserving device to maintain SpO2 at ≥ 90%, compared to continuous-flow oxygen and a standard O2-conserving device (CR-50, Puritan-Bennett, Pleasanton, California).
METHODS: We randomly assigned 28 patients who were on continuous home O2 for COPD to use each of 3 O2 delivery systems (continuous-flow O2, CR-50, and AccuO2) for 8 hours a day, for 2 consecutive days, at home, at their current O2 prescription. We recorded SpO2 and calculated the conservation ratio (duration of a given O2 supply with an O2-conserving device compared to continuous-flow O2).
RESULTS: Twenty-two patients completed all 3 study arms; 2 additional patients completed the AccuO2 arm and the continuous-flow O2 arm. The mean ± SD SpO2 was 92 ± 4% with continuous-flow O2, 92 ± 4% with the CR-50, and 91 ± 2% with AccuO2 (P = .006 for the AccuO2 vs continuous-flow O2, P = .03 for the AccuO2 vs the CR-50). SpO2 variability was less with the AccuO2 (P < .001 vs continuous-flow O2 and vs the CR-50). The conservation ratios were 9.9 ± 7.3 for the AccuO2 and 2.6 ± 1.0 for the CR-50 (P < .001).
CONCLUSIONS: Compared to continuous-flow O2 or the CR-50, the AccuO2 maintained SpO2 closer to the target, and AccuO2 had a higher conservation ratio than CR-50.
Introduction
Chronic obstructive pulmonary disease (COPD) is estimated to afflict 24 million people in the United States and is the fourth leading cause of death.1 In 2006 in the United States there were over 124,000 deaths attributable to COPD and allied conditions, 670,000 hospitalizations, and 16.3 million physician office visits.1 The total economic cost of COPD for 2010 in the United States is projected to be $49.9 billion,1 and long-term oxygen therapy (LTOT) accounts for a substantial portion of that cost. Medicare expenditures for LTOT exceed $2 billion annually.2 Portable oxygen (O2) accounts for a substantial portion of the provider cost of LTOT.3
Most LTOT systems provide a continuous fixed flow of O2. The prescribed O2 flow is typically based on a single measurement and aimed at keeping SpO2 ≥ 90%.4 O2-conserving devices are added to portable LTOT systems for patient convenience and to reduce O2 waste. The available continuous-flow and O2-conserving systems are not designed to monitor and maintain SpO2 ≥ 90% on a minute-to-minute basis. We tested a prototype oximetry-driven O2-conserving device, the AccuO2 (OptiSat Medical, Minneapolis, Minnesota) (Fig. 1), against standard continuous-flow O2 and a standard O2-conserving device (CR-50, Puritan-Bennett, Pleasanton, California).
The AccuO2 closed-loop oximetry-driven portable O2-conserving device.
Methods
This study was approved by the Minneapolis Veterans Affairs Medical Center Human Studies Committee. Written, informed consent was obtained from all subjects. The study was performed at the Minneapolis Veterans Affairs Medical Center between 1999 and 2000 and was therefore not subject to the requirement for clinic trial registration. Dr Rice had full access to all the study data and takes full responsibility for the integrity and accuracy of the data analysis.
Subjects
Via telephone, we recruited patients receiving LTOT for COPD from the Minneapolis Veterans Affairs Medical Center home O2 roster. According to the policy of the medical center, all the patients were originally prescribed LTOT to achieve a target SpO2 of 90–92%. The main inclusion criterion was a current LTOT prescription at a flow of 1–3 L/min for 18–24 hours per day. Exclusion criteria were current tobacco smoking, hospitalization for any reason within the last month, any respiratory drug prescription change in the previous 2 weeks, non-ambulatory status, active alcohol or drug addiction, any unstable disease state, and LTOT prescribed for conditions other than COPD.
Oxygen Delivery Systems
With the continuous-flow system or the CR-50, O2 was delivered via nasal cannula at the flow previously prescribed for that patient to maintain SpO2 > 90%. The AccuO2 (see Fig. 1) is designed to maintain SpO2 at a selected target at all times. The outputs of the pulse oximeter and inhalation sensor are continuously monitored by the microcontroller. The volume of O2 to be delivered is calculated by a modified proportional integral differential control algorithm, based on the difference between the observed and desired SpO2 values and the trend in that difference. Oximeter data and O2 pulse/bolus size are measured and updated every second. Starting from a steady-state situation (ie, the patient's SpO2 is stable at the target), the AccuO2 begins increasing the O2 dose on the first inhalation after a valid SpO2 reading below the target (ie, within 1–2 s). Signal averaging built into the oximeter gives an overall time constant of about 5–10 seconds. The oximeter's output includes error flags for a detached sensor, low-perfusion state, and heart rates of < 40 beats/min and > 180 beats/min. If an error condition occurs, the AccuO2 continues to deliver O2 during every inhalation at the same level administered prior to the error condition for 15 seconds. If the error condition persists for > 15 seconds the AccuO2 defaults to a standard fixed-bolus, prescription-equivalent, pulse-delivery mode (33 mL/breath bolus). The maximum O2 bolus was set at 66 mL/breath (equivalent to 4 L/min continuous flow). All the O2 used in this study was in E-size cylinders.
In-Clinic Testing
In baseline testing with each patient we confirmed the ability of the continuous-flow O2 system and the CR-50 to achieve SpO2 ≥ 90%, and the ability of each patient to trigger the CR-50. We tested several prototypes of the AccuO2 in the clinic to determine the appropriate proportional integral differential control parameters and to verify and refine the respiration-sensing algorithm. To determine O2 savings and verify that the prototype AccuO2 maintained the target SpO2, we tested the phase-1 prototype and proportional integral differential control parameters under supervision in the clinic, with patients at rest and while walking at their own pace for 15-min periods, if possible, to mimic home activities. The final version of the AccuO2, built by a contract manufacturer, was tested in the clinic with a finger oximetry sensor (8000AA-3, Nonin Medical, Plymouth, Minnesota) for up to 6 hours, during which time the patients were encouraged to mimic their usual activity levels by walking at their own pace within the Minneapolis Veterans Affairs Medical Center. After reviewing the preliminary results, our institutional review board gave further approval for the in-home testing protocol.
In-Home Testing
Patients were studied on their standing O2 prescriptions, all of which were ≤ 3 L/min. We asked the patients to use each O2 system, in random order, at home, for 8 hours a day, during daytime hours only, on 2 consecutive days. We encouraged the patients to go about their normal daily activities during every study period. The target SpO2 for the AccuO2 was set at 90%. In all 3 treatment arms the patients wore a logging pulse oximeter (8500M, Nonin Medical, Plymouth, Minnesota). The average duration of home use for each O2 delivery device was based on the amount of time recorded by the oximeter.
We continuously recorded the patients' activity levels with an actigraph (Actiwatch, Philips Respironics, Murrysville, Pennsylvania) during daytime hours only. Readings above zero during any 15-second period were counted as activity.
To determine the amount of O2 used during each 2-day period, we weighed the specially marked O2 cylinders before and after each 2-day period. A full E-cylinder nominally contains 680 L of O2. The molecular weight of O2 is 31.9988 g/mole, and molar volume is 24.465 L/mole at 25°C, so 680 L of O2 weighs 889.4 g. We calculated the conservation ratio as the duration a given amount of O2 lasted with an O2-conserving device, compared to during continuous-flow O2 therapy.
Statistical Analysis
We analyzed the SpO2 values and conservation ratios with the Mann-Whitney test. Differences in standard deviations were analyzed with the 2-tailed Kolmogorov-Smirnov test. We report mean ± SD values.
Results
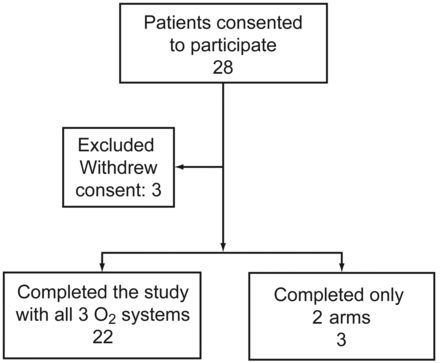
Twenty-eight patients (all male, mean age 72.3 y, age range 60–81 y) consented to participate in the in-home study (Fig. 2), but 3 of the 28 withdrew consent before testing any device. Twenty-two patients completed all 3 study arms; 2 additional patients used only the AccuO2 and the continuous-flow system. One patient used only the CR-50 and continuous O2.
Flow chart.
The mean SpO2 during the in-home study periods was significantly lower with the AccuO2 (91 ± 2%) than with the CR-50 (92 ± 4%, P = .03) or continuous-flow O2 (92 ± 4, P = .006) (Fig. 3). AccuO2 appeared to reduce the amount of time spent at low SpO2, although this difference was not statistically significant (Fig. 4). The SpO2 was ≤ 88% for more than 50% of the study period in 0, 2, and 4 patients, respectively, with the AccuO2, CR-50, and continuous-flow O2. The amount of time spent with SpO2 > 90% was significantly less with the AccuO2 (see Fig. 4). SpO2 was > 95% more than 50% of the time in 0, 9, and 6 patients, respectively, with the AccuO2, CR-50, and continuous-flow O2. SpO2 variability was significantly lower with the AccuO2. The SpO2 standard deviation was significantly smaller with the AccuO2: the means of the individual patients for the AccuO2, CR-50, and continuous-flow O2 were 2.4%, 3.2%, and 3.5%, respectively (P < .001 for AccuO2 versus CR-50 or continuous-flow O2).
Individual and mean SpO2 values during 48-hour periods at home. P = .006 for AccuO2 versus continuous-flow O2, P = .03 for AccuO2 versus CR-50.
Cumulative percent of time under various SpO2 levels during two 8-hour periods on 2 consecutive days at home. P values compared to continuous-flow O2 for each 1% increment increase in SpO2 are represented by open circles for P values < .05, and by Xs for P values > .05.
The average percentage of invalid/error oximeter data across all arms and patients was 3.3 ± 2.7%, and there were no statistically significant differences between the arms. The AccuO2 applies more stringent requirements on the oximeter data to be in closed-loop mode: the average percentage of time in the fixed-dose default mode (ie, not in closed-loop) was 14.4 ± 12.1%. Twenty-one of 24 patients were in closed-loop mode more than 75% of the time with the AccuO2.
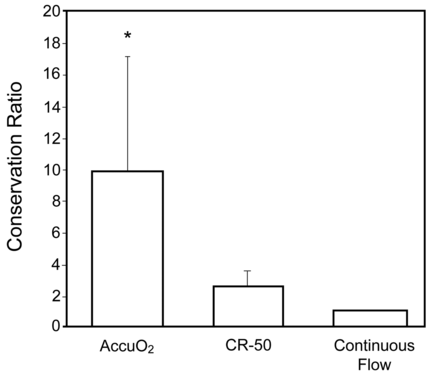
The average duration of home use was 15.5 ± 2.0 hours with the AccuO2, 14.3 ± 2.5 hours with the CR-50, and 15.8 ± 1.3 hours with the continuous-flow O2 system. Less supplemental O2 was required to maintain the target SpO2 with the AccuO2. With continuous-flow O2 as the reference, the mean conservation ratios for the AccuO2 and CR-50 were 9.9 ± 7.3 and 2.6 ± 1.0, respectively (P < .001) (Fig. 5).
Mean conservation ratios during 48-hour periods at home. *P < .001 for AccuO2 versus CR-50 and for AccuO2 versus continuous-flow O2.
The patients' daytime home activity levels were not significantly different with the different O2 systems. The average actigraphy output counts were: AccuO2 20.5 ± 59.8, CR-50 22.0 ± 65.3, and continuous-flow O2 26.3 ± 77.5. The mean percentages of time spent in activity during the 8-hour study periods were: AccuO2 31.6%, CR-50 32.0%, and continuous-flow O2 32.0%.
We asked the patients to rank the O2 systems according to their preference (1 = most preferred, 3 = least preferred), and the mean scores were: AccuO2 2.1 ± 0.9, CR-50 1.7 ± 0.7, and continuous-flow O2 2.2 ± 0.8.
Discussion
The AccuO2 maintained a clinically acceptable SpO2 with less SpO2 variation and lower O2 consumption than CR-50 or continuous-flow oxygen. Mean SpO2 was lowest with AccuO2, because the AccuO2 is designed to maintain the SpO2 as close as possible to 90%, consistent with the therapeutic goal of the Nocturnal Oxygen Therapy Trial,5 in which patients with a baseline PO2 < 55 mm Hg were given supplemental O2 to achieve a PO2 range of 60 mm Hg (which approximately corresponds to an SpO2 of 90%) to 80 mm Hg. Based on the results of that trial, there is general consensus that the SpO2 goal of LTOT should be ≥ 90%. The current COPD treatment guidelines4 recommend titrating LTOT to SpO2 ≥ 90%. Available evidence does not support a higher target SpO2, as shown in a study by Gorecka and colleagues, in which a target PO2 of > 65 mm Hg in patients with less severe hypoxemia did not confer a survival benefit.6 Patients treated with continuous LTOT have been reported to spend 10–30% of their time in a hypoxic state, including both sleep and waking hours.7–10 The primary advantage of oximetry-driven O2 delivery is maintaining the SpO2 at or near 90% while reducing O2 waste.
The mean conservation ratio with the AccuO2 was more than 3 times that with the CR-50 and 9 times that with continuous-flow O2. Although we used E cylinders in this study, by extending the duration with any cylinder size, the AccuO2 would provide a similar duration benefit for patients using smaller, less cumbersome tanks, and might substantially reduce the cost of LTOT with all cylinder sizes, because portable equipment accounts for a substantial portion of LTOT costs.
Although we observed a non-significant reduction in desaturations, this observation should be considered hypothesis-generating only: further studies with sufficient power to determine this effect are needed. Because we chose a single target SpO2 of 90% for the AccuO2, we did not determine its effectiveness or O2 savings at other target SpO2 levels. The difference in O2 savings between the AccuO2 and standard O2 delivery devices might be smaller if standard O2-conserving devices were more tightly set to achieve SpO2 of 90%, although the practicality of that approach is questionable. We believe testing patients on their standing LTOT prescriptions is applicable to clinical practice, as all patients were originally prescribed LTOT to achieve a target SpO2 of 90–92%.
Limitations
Because the in-home study period was limited to 2 consecutive days, the performance of the AccuO2 over longer periods and under more varied clinical circumstances is not known. The actigraphy data did not allow us to distinguish between sleep and inactivity, because readings above zero were counted as activity. We doubt that a reading of zero indicated that the patient was asleep, however, because the actigraph readings were zero more than 60% of the time during the day. The patients' activity levels were similar across all 3 arms, so the activity differences could not account for the substantial O2 savings we observed with the AccuO2.
Although the patient survey results suggest that the AccuO2 was as well tolerated as the CR-50, more detailed information on specific aspects of patient preference, such as comfort or convenience, is needed to better determine the feasibility of the AccuO2. Options for potentially less burdensome oximeter sensors, such as a reflectance sensor on the forehead or behind the ear, an ear-lobe sensor, or wireless oximeters, should be considered for future testing.
Closed-loop, oximetry-driven O2 delivery has potential application beyond ambulatory LTOT. Almost 50% of patients on LTOT for COPD are reported to experience substantial nocturnal desaturation with standard O2 delivery systems.10 Although we did not study our patients during sleep, the AccuO2 has potential application for maintaining a therapeutic nocturnal SpO2. Oximetry-driven O2 delivery systems may also have applications in settings other than the treatment of patients with stable COPD. In patients who are hospitalized for COPD exacerbation, pulse oximetry-driven O2 delivery could be used to avoid the high SpO2 values that have been associated with acute CO2 retention. Systems driven by continuous oximetry and blood-gas data have been used to automate O2 delivery in neonates and pre-term infants.11–13
Conclusions
This study provides preliminary evidence that O2 delivery with the AccuO2 is as or more effective than standard O2 delivery systems in maintaining a target SpO2 via continuous adjustment of O2 delivery based on physiologic need. By improving O2 conservation, AccuO2 may improve patient convenience, potentially at a lower cost. Further studies should be done to confirm the observation that patients appear to spend less time at low SpO2 with AccuO2. Long-term studies, particularly in the ambulatory setting in patients with various levels of disease stability and other clinical conditions, are also needed to determine the impact of this novel O2 delivery system.
Footnotes
- Correspondence: Kathryn L Rice MD, Pulmonary Section, Minneapolis Veterans Affairs Medical Center, 1 Veterans Drive, Minneapolis MN 55417. E-mail: kathryn.rice{at}va.gov.
This research was partly supported by the University of Minnesota Technology Partnership Fund and the Minneapolis Veterans Affairs Research Service. Dr Rice has disclosed a relationship with Wyeth Pharmaceuticals. Dr Schmidt, Dr Buan, and Mr Schwarzock have disclosed relationships with OptiSat Medical. Ms Lebahn has disclosed no conflicts of interest.
Dr Rice presented a version of this paper at the 103rd International Conference of the American Thoracic Society, held May 18–23, 2007, in San Francisco, California.
See the Related Editorial on Page 1975
- Copyright © 2011 by Daedalus Enterprises Inc.