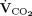Abstract
BACKGROUND: Switching patients affected by early severe ARDS and undergoing extracorporeal membrane oxygenation (ECMO) from controlled ventilation to spontaneous breathing can be either beneficial or harmful, depending on how effectively the breathing pattern is controlled with ECMO. Identifying the factors associated with ineffective control of spontaneous breathing with ECMO may advance our pathophysiologic understanding of this syndrome.
METHODS: We conducted a prospective study in subjects with severe ARDS who were on ECMO support ≤ 7 d. Subjects were switched to minimal sedation and pressure-support ventilation while extracorporeal CO2 removal was increased to approximate the subject’s total CO2 production ( ). We calculated the rapid shallow breathing index (RSBI) as breathing frequency divided by tidal volume. We explored the correlation between certain characteristics recorded during pretest controlled ventilation and the development of apnea (ie, expiratory pause lasting > 10 s; n = 3), normal breathing pattern (ie, apnea to RSBI ≤ 105 breaths/min/L; n = 6), and rapid shallow breathing (RSBI > 105 breaths/min/L; n = 6) that occurred during the test study.
). We calculated the rapid shallow breathing index (RSBI) as breathing frequency divided by tidal volume. We explored the correlation between certain characteristics recorded during pretest controlled ventilation and the development of apnea (ie, expiratory pause lasting > 10 s; n = 3), normal breathing pattern (ie, apnea to RSBI ≤ 105 breaths/min/L; n = 6), and rapid shallow breathing (RSBI > 105 breaths/min/L; n = 6) that occurred during the test study.
RESULTS: The ratio of extracorporeal CO2 removal to the subjects’  was >90% in all 15 subjects, and arterial blood gases remained within normal ranges. Baseline pretest Sequential Organ Failure Assessment score, total
was >90% in all 15 subjects, and arterial blood gases remained within normal ranges. Baseline pretest Sequential Organ Failure Assessment score, total  and ventilatory ratio increased steadily, whereas
and ventilatory ratio increased steadily, whereas 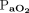 /
/ was higher in subjects with apnea compared to intermediate RSBI ≤105 breaths/min/L and elevated RSBI >105 breaths/min/L. In subjects with rapid shallow breathing, baseline lung weight measured with quantitative computed tomography scored higher, as well.
was higher in subjects with apnea compared to intermediate RSBI ≤105 breaths/min/L and elevated RSBI >105 breaths/min/L. In subjects with rapid shallow breathing, baseline lung weight measured with quantitative computed tomography scored higher, as well.
CONCLUSIONS: In early severe ARDS, the factors associated with rapid shallow breathing despite maximum extracorporeal CO2 extraction include less efficient CO2 and O2 exchange by the natural lung, higher severity of organ failure, and greater magnitude of lung edema.
Introduction
In patients affected by ARDS, spontaneous breathing can result in multiple physiological benefits, such as improved ventilation-perfusion matching, preserved respiratory muscle function, and decreased need for sedatives.1 However, during the early acute phase of severe ARDS, the patient’s breathing pattern might be overactivated by a variety of factors, including derangement of arterial blood gases, activation of lung receptors by excessive edema, or non-physiological displacement of the chest wall.2,3 A combination of these conditions may cause an uncontrolled breathing pattern, which increases the risk of lung and diaphragm injury.4,5 In severe ARDS, early switching to spontaneous respiratory activity while monitoring the breathing pattern may provide the physiological benefits described above and also avoid additional injury.6
Extracorporeal membrane oxygenation (ECMO) is widely used in patients with severe ARDS who are unresponsive to conventional treatment.7,8 In addition to improving oxygenation, ECMO allows effective removal of CO2, which is expected to control the respiratory drive and facilitate protective spontaneous breathing.9-11 Previous studies have reported that ECMO can reduce the breathing frequency to apnea in healthy lungs or to quiet breathing in subjects recovering from severe ARDS.11,12 On the other hand, pilot observations have noted that subjects affected by early severe ARDS might not achieve a safe spontaneous breathing pattern despite the presence of ECMO support.13,14 None of the studies performed during early ARDS explored the correlation between pretest physiology and the breathing pattern during maximum extracorporeal CO2 removal, nor did they describe the relationship between control of the breathing pattern by CO2 removal and clinical outcomes.
In this prospective pilot study, we analyzed the spontaneous breathing pattern during maximum extracorporeal CO2 removal in intubated subjects with early severe ARDS on ECMO, with the purpose of identifying subjects in whom spontaneous breathing pattern was poorly controlled with ECMO, describing pretest physiological characteristics associated with failure of breathing control, and exploring associations between breathing control and clinical outcomes.
Our hypothesis was that subjects with poor control over their breathing pattern might be characterized by more severe baseline lung injury and worse clinical outcomes.13 If proven true, our findings might inform a larger clinical study aimed at establishing a safe protocol to switch to spontaneous breathing in subjects affected by early severe ARDS undergoing ECMO.
QUICK LOOK
Current knowledge
Extracorporeal membrane oxygenation provides oxygenation and allows CO2 removal, which could control the respiratory pattern of patients with ARDS. However, pilot observations indicate that, in subjects with early severe ARDS, CO2 removal may fail to control the spontaneous breathing pattern.
What this paper contributes to our knowledge
In subjects with early severe ARDS, maximum CO2 removal led to high interindividual variability in terms of spontaneous breathing pattern. The factors associated with poor control of the respiratory pattern during pretest baseline conditions were 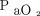 /
/ , ventilatory ratio, the SOFA score, the subject’s total CO2 production, and the lung weight measured with computed tomography. All of these factors indicated more severe lung injury and greater systemic involvement.
, ventilatory ratio, the SOFA score, the subject’s total CO2 production, and the lung weight measured with computed tomography. All of these factors indicated more severe lung injury and greater systemic involvement.
Methods
Population
The study was conducted within the intensive care unit of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, in Milan, Italy. It was approved by the Institutional Ethics Committee (reference number 1496). Informed consent was obtained from each subject according to local regulations. All consecutive, intubated, sedated, and paralyzed patients with severe ARDS on venovenous ECMO were screened for enrollment in the study between October 2016 and February 2018. Paralysis is a usual practice in our center for patients with severe ARDS during the first days they are connected to ECMO.
Subjects with severe ARDS on venovenous ECMO support (Maquet CARDIOHELP device with HLS Set Advanced or Maquet PLS membrane oxygenator with Rotaflow pump and console; Getinge Italia, Cinisello Balsamo, Italy) for at least 48 h and ≤ 7 d were included. Patients with > 7 d of intubation before ECMO start, hemodynamic instability (mean arterial blood pressure < 60 mm Hg despite vasoactive drugs), pulmonary plasma leakage, alveolar bleeding, moribund status, or refusal by the physician in charge were excluded.
Data Collection
The following data were collected under clinical settings before starting (ie, pretest conditions): demographic data, ARDS etiology, Sequential Organ Failure Assessment (SOFA) score, controlled mechanical ventilation and ECMO settings, respiratory mechanics, arterial and mixed venous blood gases, and intrapulmonary shunt fraction according to standard equation. Physiological dead space was also calculated pretest, using the Enghoff modification of the Bohr equation.15,16 A modified ventilatory ratio17 was calculated as:

Lung CT scans performed between intubation and enrollment for clinical reasons were quantitatively analyzed to measure total and regional lung weight as previously described.18 Regional quantitative analysis of the dependent and nondependent regions of the lung was performed by dividing in half each contoured area of the CT scan sectional images.
Lastly, the total number of days on ventilation, days spent on controlled mechanical ventilation until day 28 from intubation, total ECMO duration, and hospital mortality were collected.
Study Protocol
A nasogastric tube equipped with an integrated esophageal balloon (Nutrivent, Sidam, Mirandola, Italy) was used to measure esophageal pressure (Pes) by the standard method.19-21 A combined infrared CO2 and flow sensor was inserted between the Y-piece of the circuit ventilator and the endotracheal tube. This sensor was connected to a volumetric capnograph (CO2SMO Plus, Novametrix Medial, Wallington, Connecticut) to measure natural lung 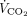 . Hemodynamics (invasive arterial pressure and heart rate), peripheral oxygen saturation and
. Hemodynamics (invasive arterial pressure and heart rate), peripheral oxygen saturation and  of the natural lung were monitored at all times.
of the natural lung were monitored at all times. 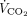 removed with ECMO was calculated by multiplying the mean CO2 concentration in the gas exiting the membrane lung by the rate of sweep gas flow.13 Each subject’s total
removed with ECMO was calculated by multiplying the mean CO2 concentration in the gas exiting the membrane lung by the rate of sweep gas flow.13 Each subject’s total 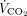 was calculated as the sum of the
was calculated as the sum of the  of the natural lung and
of the natural lung and 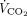 removed with ECMO).12,13
removed with ECMO).12,13
The study consisted of 5 phases. First, sedative drugs and neuromuscular blocking agents were discontinued. The subject achieved a stable grade on the Richmond Agitation Sedation Scale between −2 and 0. Evidence of inspiratory trigger and no residual paralysis could be then ascertained. Second, subjects were switched to pressure-support ventilation (5 cm H2O or 10 cm H2O in subjects with tidal volume < 100 mL) while maintaining clinical PEEP and 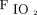 .
.
Third, ECMO sweep gas flow with 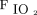 1.0 was steadily increased to reach
1.0 was steadily increased to reach  of the natural lung ≤ 20 mL/min as measured with the volumetric capnography monitor. This value was identified as a suitable working compromise for the high probability of obtaining maximum CO2 removal by ECMO (ie,
of the natural lung ≤ 20 mL/min as measured with the volumetric capnography monitor. This value was identified as a suitable working compromise for the high probability of obtaining maximum CO2 removal by ECMO (ie,  removed with ECMO >90% of total
removed with ECMO >90% of total  ). Fourth, the selected gas flow was maintained for 15 min, and, finally, data collection was performed.
). Fourth, the selected gas flow was maintained for 15 min, and, finally, data collection was performed.
During phase 5, the waveforms of Pes, airway pressure with an end-expiratory hold, and flow were recorded. Arterial blood gas analysis, 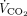 of the natural lung, and
of the natural lung, and  removed with ECMO were measured. The following data were gathered from offline analysis: tidal volume, breathing frequency (measured as the number of negative deflections of Pes in a minute), the presence of pauses in the breathing frequency > 10 s, minute ventilation, airway-occlusion pressure 0.1 s after the start of inspiration against an occluded airway, and driving transpulmonary pressure. After data collection, deep sedation, paralysis, pretest controlled ventilation, and ECMO settings were promptly restored.
removed with ECMO were measured. The following data were gathered from offline analysis: tidal volume, breathing frequency (measured as the number of negative deflections of Pes in a minute), the presence of pauses in the breathing frequency > 10 s, minute ventilation, airway-occlusion pressure 0.1 s after the start of inspiration against an occluded airway, and driving transpulmonary pressure. After data collection, deep sedation, paralysis, pretest controlled ventilation, and ECMO settings were promptly restored.
Characteristics of the Breathing Pattern
For each subject, using the data measured offline, we calculated the rapid shallow breathing index (RSBI) as the ratio between breathing frequency and tidal volume.22 Subjects were grouped into 3 categories:
(1) Subjects developing apnea (ie, absence of inspiratory Pes deflections for > 10 s);
(2) Subjects with normal breathing pattern (ie, RSBI ≤ 105 breaths/min/L)
(3) Subjects with rapid shallow breathing (ie, RSBI > 105 breaths/min/L).
Statistical Analysis
Categorical variables are reported as frequency (percentage); continuous variables are reported as median (interquartile range). Comparisons between groups were performed with the chi-square or Fisher exact test for categorical variables, and with the Kruskal-Wallis nonparametric test for continuous variables. To disclose baseline pretest variables associated with increasingly unphysiological breathing patterns, the Spearman correlation coefficient was calculated. The sample size was similar to previous studies.12,13 P values < .05 were considered statistically significant.
Results
Population
We screened 23 consecutive patients between October 2016 and February 2018, and 15 subjects were enrolled in the study. Eight patients were excluded due to hemodynamic instability (n = 3), pulmonary plasma leakage (n = 2), alveolar bleeding (n = 1), or organizational reasons (n = 2). Characteristics, clinical severity, baseline ventilation, and ECMO settings of the enrolled subjects before commencement of the study protocol are reported in Table 1. Subjects were enrolled after a median of 5 d (4–8) from intubation and 3 d –6) of ECMO. Total duration of ECMO support was 13 d (6–9), and hospital mortality was 20% (n = 3 subjects).
Baseline Pretest Characteristics of the Study Population
Dynamic Respiratory Response to Maximum  Removal With ECMO
Removal With ECMO
Ventilation settings, gas exchange, and breathing pattern during phase 5 are reported in Table 2. A single, well-defined breathing pattern was recognized in each subject. Despite maximum  removal with ECMO [ie, median ratio of
removal with ECMO [ie, median ratio of 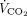 removed with ECMO to total
removed with ECMO to total 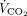 , obtained during study phase 4 was 99.7% (98.0–100) with ECMO gas flow of12.0 L/min (8.5–17.5)] and almost normal levels of
, obtained during study phase 4 was 99.7% (98.0–100) with ECMO gas flow of12.0 L/min (8.5–17.5)] and almost normal levels of 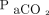 , pH, and
, pH, and  , apnea occurred only in 3 of 15 subjects (20%). Six subjects (40%) presented normal breathing pattern with RSBI ≤ 105 breaths/min/L, and 6 subjects (40%) developed rapid shallow breathing with RSBI > 105 bpm/L. Among the variables measured during the test, only respiratory frequency increased in subjects with less physiological control over breathing (Table 2). Remarkably, during the test, the ratio between
, apnea occurred only in 3 of 15 subjects (20%). Six subjects (40%) presented normal breathing pattern with RSBI ≤ 105 breaths/min/L, and 6 subjects (40%) developed rapid shallow breathing with RSBI > 105 bpm/L. Among the variables measured during the test, only respiratory frequency increased in subjects with less physiological control over breathing (Table 2). Remarkably, during the test, the ratio between 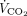 extraction with the ECMO system and the subject’s total
extraction with the ECMO system and the subject’s total  stayed constant across the 3 groups.
stayed constant across the 3 groups.
Parameters Measured During Spontaneous Breathing and Maximum Extracorporeal 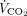 Removal With ECMO
Removal With ECMO
Pretest Baseline Variables Correlated with Spontaneous Breathing Patterns
The correlations between baseline characteristics collected during controlled ventilation before the start of the study protocol and the 3 spontaneous breathing patterns were evaluated. Variables showing worse values in subjects with higher RSBI (Fig. 1) were the SOFA score and the subject’s total CO2 production (both indicating greater clinical severity); 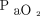 /
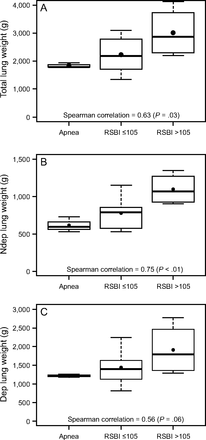
/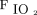 ; and the ventilatory ratio (2 indexes of less efficient oxygenation and elimination of CO2 by the diseased lungs). Moreover, in the CT scan analysis (n = 12), subjects with rapid shallow breathing showed greater total lung weight due to excessive edema in the nondependent regions (Fig. 2). Interestingly, traditional indexes of ARDS severity, such as lower respiratory system compliance, higher driving pressure, higher dead space, and larger intrapulmonary shunt, weren’t correlated with the development of a more rapid shallow breathing pattern (P > .05 for all).
; and the ventilatory ratio (2 indexes of less efficient oxygenation and elimination of CO2 by the diseased lungs). Moreover, in the CT scan analysis (n = 12), subjects with rapid shallow breathing showed greater total lung weight due to excessive edema in the nondependent regions (Fig. 2). Interestingly, traditional indexes of ARDS severity, such as lower respiratory system compliance, higher driving pressure, higher dead space, and larger intrapulmonary shunt, weren’t correlated with the development of a more rapid shallow breathing pattern (P > .05 for all).
During maximal extra-corporeal CO2 extraction, (from left to right) in subjects developing apnea, with a normal breathing pattern and RSBI ≤105, and with rapid shallow breathing and RSBI >105 (see text for details), the SOFA score and the total subject’s CO2 production increased (both indicating worse clinical severity), the PaO2/FIO2 ratio decreased and the ventilatory ratio increased (indicating less efficient lung function). Data are presented as 10th, 25th, 50th, 75th, and 90th percentiles with box plots. Correlations were tested with Spearman nonparametric coefficients. SOFA = Sequential Organ Failure Assessment; RSBI = rapid shallow breathing index.
Global and regional lung weight measured with computed tomography in subjects with complete, partial, or poor control of breathing by CO2 removal. Subjects developing apnea showed lower lung weight in comparison to subjects with RSBI ≤ 105, and subjects with RSBI > 105 showed the highest lung weight. Notably, difference in lung weight was more evident in the nondependent lung regions. Data are presented as 10th, 25th, 50th, 75th, and 90th percentiles by box plots. Correlations were tested with Spearman nonparametric coefficients. RSBI = rapid shallow breathing index.
Clinical Outcomes
The median number of days free from controlled mechanical ventilation at day 28 was 23 d (0–26) in subjects with apnea, 18 d (14–23) in subjects with RSBI ≤ 105 breaths/min/L, and 2 d (0–13) in subjects with rapid shallow breathing (P = .10). The total duration of mechanical ventilation was 19 d (10–55) versus 14 d (11–48) versus 39 d (28–61) for apnea versus RSBI ≤105 breaths/min/L versus RSBI > 105 breaths/min/L (P = .43); duration of ECMO support was 7 d (5–17) versus 10 d (6–39) versus 28 d (10–47) for apnea versus RSBI ≤ 105 breaths/min/L versus RSBI > 105 breaths/min/L (P = .36). Lastly, 12 (80%) subjects were successfully disconnected from ECMO, completed weaning, and survived. Hospital mortality rates within the study population were 0 of 3 (0%) subjects versus 0 of 6 (0%) subjects versus 3 of 6 (50%) subjects for apnea versus RSBI ≤ 105 breaths/min/L versus RSBI > 105 breaths/min/L (P = .17).
Discussion
The key findings of this pilot study can be summarized as follows: subjects with early severe ARDS undergoing maximum 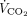 removal with ECMO exhibit extremely high interindividual variability in terms of spontaneous breathing pattern, with a group developing uncontrolled rapid shallow breathing. During baseline controlled ventilation, poor control of spontaneous breathing despite maximum
removal with ECMO exhibit extremely high interindividual variability in terms of spontaneous breathing pattern, with a group developing uncontrolled rapid shallow breathing. During baseline controlled ventilation, poor control of spontaneous breathing despite maximum 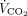 removal may be predicted by faulty O2 and CO2 exchange by the natural lung, more severe systemic involvement, or greater lung weight on CT scan analysis. Subjects with poor control of spontaneous breathing by
removal may be predicted by faulty O2 and CO2 exchange by the natural lung, more severe systemic involvement, or greater lung weight on CT scan analysis. Subjects with poor control of spontaneous breathing by  removal may have increased mortality and may not tolerate early transition to assisted breathing.
removal may have increased mortality and may not tolerate early transition to assisted breathing.
The risk of developing additional self-inflicted lung injury hinders spontaneous breathing in patients affected by early severe ARDS. Indeed, in cases of severe ARDS, controlled mechanical ventilation is recommended to avoid the risk of high respiratory drive, which worsens lung injury through uncontrolled spontaneous breathing patterns.5 Fine tuning of the spontaneous respiratory pattern by 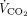 removal is an appealing alternative approach to protect the severe ARDS lung because maximum
removal is an appealing alternative approach to protect the severe ARDS lung because maximum  removal could theoretically allow a safe spontaneous breathing pattern.23 However, accumulating evidence indicates that the inverse linear relationship between extracorporeal
removal could theoretically allow a safe spontaneous breathing pattern.23 However, accumulating evidence indicates that the inverse linear relationship between extracorporeal  removal with ECMO and minute ventilation might be impaired in patients with early severe ARDS. Preclinical data in an animal model of severe ARDS indicated that a subgroup of animals had only a minimal decrease of their minute ventilation despite the
removal with ECMO and minute ventilation might be impaired in patients with early severe ARDS. Preclinical data in an animal model of severe ARDS indicated that a subgroup of animals had only a minimal decrease of their minute ventilation despite the  removed with ECMO being ≥90% of total
removed with ECMO being ≥90% of total 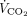 .24 In clinical settings, ECMO allowed almost linear control of the spontaneous breathing pattern in subjects recovering from severe ARDS12,25,26; however, persistently heightened respiratory frequency and increased effort despite substantial
.24 In clinical settings, ECMO allowed almost linear control of the spontaneous breathing pattern in subjects recovering from severe ARDS12,25,26; however, persistently heightened respiratory frequency and increased effort despite substantial  removal with ECMO were described in subjects with early severe ARDS.13,14 Thus, in the present study, we further explored full breathing control in subjects with early severe ARDS through maximum
removal with ECMO were described in subjects with early severe ARDS.13,14 Thus, in the present study, we further explored full breathing control in subjects with early severe ARDS through maximum 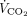 extraction with ECMO, confirming the high degree of interindividual variability. The range of respiratory breathing patterns was extremely wide despite similarly high ECMO support and normal arterial blood gases: only 3 subjects reached apnea, 40% of subjects exhibited a normal breathing pattern, and 40% of subjects developed uncontrolled rapid shallow breathing. The fact that rapid shallow breathing resulted mostly from high respiratory frequency may indicate that poor respiratory system mechanics and muscular weakness limited the subjects’ ability to increase tidal volume.6 Lack of control of the spontaneous breathing pattern with ECMO most likely highlights our limited understanding of severe ARDS patho-physiology, because radically different phenotypes might coexist under the same diagnosis.27-30
extraction with ECMO, confirming the high degree of interindividual variability. The range of respiratory breathing patterns was extremely wide despite similarly high ECMO support and normal arterial blood gases: only 3 subjects reached apnea, 40% of subjects exhibited a normal breathing pattern, and 40% of subjects developed uncontrolled rapid shallow breathing. The fact that rapid shallow breathing resulted mostly from high respiratory frequency may indicate that poor respiratory system mechanics and muscular weakness limited the subjects’ ability to increase tidal volume.6 Lack of control of the spontaneous breathing pattern with ECMO most likely highlights our limited understanding of severe ARDS patho-physiology, because radically different phenotypes might coexist under the same diagnosis.27-30
To account for the intrinsic heterogeneity of early severe ARDS, pretest identification of subjects for whom ECMO could allow effective control of spontaneous breathing may be clinically crucial because an early switch to assisted breathing might be dangerous in nonresponders and beneficial in responders. In this study, increasingly nonphysiologic breathing patterns during maximum ECMO support were associated with lower pretest oxygenation efficiency, higher weighted minute ventilation needed to maintain normal CO2 levels (ie, the ventilatory ratio), more severe organ dysfunction, more intense systemic metabolic activation, and more extensive and diffuse lung edema. Our findings suggest 2 directions on the pathophysiological understanding of severe ARDS.
On one hand, the intrinsic severity of lung injury could represent a direct and independent trigger for respiratory drive. Whereas  /
/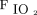 is a stratification factor in ARDS by definition, ventilatory ratio is a relatively new and interesting marker of ARDS severity.15,31 In the early phase of ARDS, poor efficiency of CO2 extraction by the natural lung is mainly due to diffuse microvascular injury and occlusion of ventilated units deriving from overactivation of coagulation by the inflammatory processes.32,33 Likewise, an increase in lung weight as measured with CT is a direct quantification of the spreading of alveolar inflammatory lung edema, which is probably the most sensitive measure of the “core” extent of ARDS severity.18,34,35 The nondependent lung is usually spared the most from consolidations (ie, the baby lung), and it seems reasonable that our finding of higher lung edema in this region indicates more severe lung injury.18 In this context, the exaggerated activation of pulmonary receptors due to increasingly severe forms of ARDS could be responsible for an uncontrolled respiratory pattern independent of CO2 removal.34-37
is a stratification factor in ARDS by definition, ventilatory ratio is a relatively new and interesting marker of ARDS severity.15,31 In the early phase of ARDS, poor efficiency of CO2 extraction by the natural lung is mainly due to diffuse microvascular injury and occlusion of ventilated units deriving from overactivation of coagulation by the inflammatory processes.32,33 Likewise, an increase in lung weight as measured with CT is a direct quantification of the spreading of alveolar inflammatory lung edema, which is probably the most sensitive measure of the “core” extent of ARDS severity.18,34,35 The nondependent lung is usually spared the most from consolidations (ie, the baby lung), and it seems reasonable that our finding of higher lung edema in this region indicates more severe lung injury.18 In this context, the exaggerated activation of pulmonary receptors due to increasingly severe forms of ARDS could be responsible for an uncontrolled respiratory pattern independent of CO2 removal.34-37
On other hand, systemic involvement deriving from the spread of pulmonary inflammation could limit the role of arterial blood gas values in controlling respiratory drive.38 In our subjects, greater involvement of systemic organs quantified by the SOFA score and higher total  (ie, a hypermetabolic state, as suggested also by a higher ventilatory ratio) could indicate that ARDS wasn’t “compartmentalized” enough, suggesting a link between severity of lung injury and the systemic spread of detrimental lung mediators, which has already been described.39 In this context, derangements of regional pH and tissue CO2 values could have overridden the respiratory drive to develop nonphysiologic breathing patterns. Lastly, our exploratory observation that subjects developing rapid shallow breathing exhibited a delayed switch to assisted ventilation and a higher mortality rate may suggest that the proposed test can identify a more critical subgroup of patients with early severe ARDS. This reasoning should be considered as hypothesis-generating, given the pilot nature of our study and considering the limited experience and experimental data available on the topic, as well as the wide reported interindividual variability. Still, a brief test of spontaneous breathing under maximum extracorporeal
(ie, a hypermetabolic state, as suggested also by a higher ventilatory ratio) could indicate that ARDS wasn’t “compartmentalized” enough, suggesting a link between severity of lung injury and the systemic spread of detrimental lung mediators, which has already been described.39 In this context, derangements of regional pH and tissue CO2 values could have overridden the respiratory drive to develop nonphysiologic breathing patterns. Lastly, our exploratory observation that subjects developing rapid shallow breathing exhibited a delayed switch to assisted ventilation and a higher mortality rate may suggest that the proposed test can identify a more critical subgroup of patients with early severe ARDS. This reasoning should be considered as hypothesis-generating, given the pilot nature of our study and considering the limited experience and experimental data available on the topic, as well as the wide reported interindividual variability. Still, a brief test of spontaneous breathing under maximum extracorporeal  removal could be proposed as a screening test to assess control of spontaneous breathing pattern in individual subjects.
removal could be proposed as a screening test to assess control of spontaneous breathing pattern in individual subjects.
This study has several limitations. First, the short duration of the study protocol presented safety advantages, but it may limit its validity because spontaneous breathing patterns might have been affected by residual sedation and too little time to reach stable physiological conditions. Nevertheless, arterial gas exchange already reached normal values, thus limiting the influence of the hypoxic and hypercapnic drive during the test. Second, the study protocol was performed under pressure-support ventilation, but other modes of assisted ventilation (eg, proportional modes) could have affected breathing patterns differently.12,37 Third, volumetric capnography is currently the most widely used method to measure the  of the natural lung, but we must acknowledge that the dynamic nature of the measurements we took with very small and variable tidal volumes might underestimate the
of the natural lung, but we must acknowledge that the dynamic nature of the measurements we took with very small and variable tidal volumes might underestimate the  exhaled through the natural lung.
exhaled through the natural lung.
Conclusions
Response to maximum extracorporeal  removal with ECMO in terms of control of spontaneous breathing in subjects with early severe ARDS presented high interindividual variability, with a subgroup clearly characterized by uncontrolled rapid shallow breathing. Failure to control the breathing pattern with CO2 removal might be associated with less efficient O2 delivery and CO2 elimination by the natural lung, more serious organ failure, and systemic metabolic activation, as well as by increased lung weight.
removal with ECMO in terms of control of spontaneous breathing in subjects with early severe ARDS presented high interindividual variability, with a subgroup clearly characterized by uncontrolled rapid shallow breathing. Failure to control the breathing pattern with CO2 removal might be associated with less efficient O2 delivery and CO2 elimination by the natural lung, more serious organ failure, and systemic metabolic activation, as well as by increased lung weight.
Footnotes
- Correspondence: Tommaso Mauri MD, Department of Anesthesia, Critical Care and Emergency, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Via F. Sforza 35, 20122 Milan, Italy. E-mail: tommaso.mauri{at}unimi.it
Dr Grasselli has disclosed relationships with Getinge, Draeger Medical, Fisher & Paykel, Pfizer, and Biotest. Dr Pesenti has disclosed relationships with Maquet, Novalung/Xenios, Baxter, and Boehringer Ingelheim. This work was supported in part by institutional funding from the Department of Anesthesia, Critical Care and Emergency, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy. The remaining authors have disclosed no conflicts of interest.
SEE THE RELATED EDITORIAL ON PAGE 1057
- Copyright © 2020 by Daedalus Enterprises