Respiratory compromise is defined as a deterioration in respiratory function such that timely interventions are needed to prevent intubation or death.1 In this month's issue, Bedoya et al2 highlights a significant problem in hospitals today. Patients on general medical floors, often without continuous monitoring, are at risk for respiratory decompensation arising from progressing illness and opioid medications. The authors report incidence rates of 0.394–0.430 per 1,000 bed-days in their community and academic hospitals, which extrapolates to approximately 64,000 events annually in the United States.2 Those patients requiring unplanned intubations had a mortality rate of 49.1%.2 These unplanned intubations and mortality are resulting from complications that could be preventable, if recognized in a timely manner.
A recent article in Respiratory Care by Lamberti1 nicely explains respiratory compromise and describes issues related to monitoring patients in the general care units. Lamberti1 describes 6 pathophysiologic changes that can result in respiratory compromise: respiratory depression due to opioids or injury to the central nervous system, central or obstructive sleep apnea, parenchymal lung disease resulting in decrease compliance and increased right to left shunting, COPD or asthma exacerbations, pulmonary edema due to fluid overload, and right heart failure due to pulmonary hypertension. Each of these conditions has different clinical presentations and requires clinicians to identify different physiologic changes to recognize them.
These pathophysiologic changes can lead to unexpected hospital mortality through 3 different patterns or trajectories.3 Type 1 is most common and is described as hyperventilation due to hypoxemia or metabolic acidosis. This pattern is associated with disorders such as sepsis, pulmonary embolism, or congestive heart failure. The hyperventilation provides the appearance of a stable 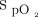 . As the metabolic acidosis progresses and the patient fatigues,
. As the metabolic acidosis progresses and the patient fatigues,  begins to fall late in the process. If supplemental oxygen is provided,
begins to fall late in the process. If supplemental oxygen is provided,  may not fall until right before the patient arrests. Type 2 is described as hypoventilation due to respiratory center depression from narcotic administration. This fall in minute volume leads to a progressive increase in
may not fall until right before the patient arrests. Type 2 is described as hypoventilation due to respiratory center depression from narcotic administration. This fall in minute volume leads to a progressive increase in  . As the CO2 level climbs, it displaces oxygen in alveoli and causes a slow decline in
. As the CO2 level climbs, it displaces oxygen in alveoli and causes a slow decline in  . Elevated
. Elevated 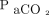 levels decrease pH, shifting the oxygen dissociation curve to the left. This leftward shift provides a higher saturation reading for a given
levels decrease pH, shifting the oxygen dissociation curve to the left. This leftward shift provides a higher saturation reading for a given 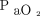 , further masking the developing acidosis and hypoxemia. Similar to Type 1, if the patient is provided supplemental oxygen,
, further masking the developing acidosis and hypoxemia. Similar to Type 1, if the patient is provided supplemental oxygen, 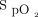 may not fall until right before decompensation. Type 3 is described as repetitive air-flow obstructions and desaturations followed by an arousal failure and resulting in sudden death. During sleep apnea, the obstruction of the upper airway leads to an elevation in
may not fall until right before decompensation. Type 3 is described as repetitive air-flow obstructions and desaturations followed by an arousal failure and resulting in sudden death. During sleep apnea, the obstruction of the upper airway leads to an elevation in 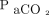 and a drop in
and a drop in 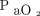 , which results in a patient arousal to clear the obstruction and a brief period of hyperventilation. If a patient with sleep apnea fails to have this arousal response, severe hypoxemia develops, leading to arrest. This arousal threshold is lowered with the use of narcotics or sedatives, which can increase the occurrence of this type of arrest.3
, which results in a patient arousal to clear the obstruction and a brief period of hyperventilation. If a patient with sleep apnea fails to have this arousal response, severe hypoxemia develops, leading to arrest. This arousal threshold is lowered with the use of narcotics or sedatives, which can increase the occurrence of this type of arrest.3
Because 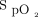 may not fall until late in the process, how do we identify these patients at risk for respiratory compromise? Breathing frequency, heart rate, age, and systolic blood pressure have been identified as the most important predictors of cardiac arrest, death on the wards, or transfer to ICU.4 The risks for these outcomes were associated with both high and low values for breathing frequency, heart rate, and systolic blood pressure. This U-shaped curve related to risk was more pronounced with breathing frequency, which was the most predictive factor. A more rapid increase in risk for these outcomes occurs around the age of 40 y and plateaus around 75–80 y.4
may not fall until late in the process, how do we identify these patients at risk for respiratory compromise? Breathing frequency, heart rate, age, and systolic blood pressure have been identified as the most important predictors of cardiac arrest, death on the wards, or transfer to ICU.4 The risks for these outcomes were associated with both high and low values for breathing frequency, heart rate, and systolic blood pressure. This U-shaped curve related to risk was more pronounced with breathing frequency, which was the most predictive factor. A more rapid increase in risk for these outcomes occurs around the age of 40 y and plateaus around 75–80 y.4
Bedoya et al reported that 46.3% of the subjects had no change in respiratory rate, heart rate, blood pressure, or 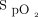 before the unplanned intubation or arrest.2 They reported that respiratory rate decreased in 4.8% and increased 13.1% in 24 hours before the event. Vitals signs were recorded intermittently and as noted in the limitations there may have been data entry discrepancies which could explain the lack of change in vital signs.2 Others have reported errors in recording vital signs5 and respiratory rate.6 More education and quality assurance efforts are needed on the importance of accurately documenting and alerting providers regarding trends in manually entered intermittent vital signs.
before the unplanned intubation or arrest.2 They reported that respiratory rate decreased in 4.8% and increased 13.1% in 24 hours before the event. Vitals signs were recorded intermittently and as noted in the limitations there may have been data entry discrepancies which could explain the lack of change in vital signs.2 Others have reported errors in recording vital signs5 and respiratory rate.6 More education and quality assurance efforts are needed on the importance of accurately documenting and alerting providers regarding trends in manually entered intermittent vital signs.
Bedoya et al2 reported that < 1% of their patients were on an end-tidal CO2 measuring device and 35.3% of patients received opioids. As noted by the authors, the providers thought that these patients were at low risk for cardiopulmonary decompensation.2 Continuous capnography monitoring is almost 6 times more likely to identify postoperative respiratory depression compared to 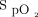 .7 Perhaps it is time to mandate the use of continuous monitoring during postoperative sedation. Challenges include the difficulty in patient compliance with wearing the device, as well as the need for provider, staff, and patient education.
.7 Perhaps it is time to mandate the use of continuous monitoring during postoperative sedation. Challenges include the difficulty in patient compliance with wearing the device, as well as the need for provider, staff, and patient education.
There exists an opportunity for respiratory therapists to take the lead in the early recognition of patients with respiratory compromise and recommend interventions to prevent intubation or arrest. Perhaps the time has come to establish continuous monitoring in high-risk patients and to collaborate with information technology departments to push alerts to clinicians in real time when observed combinations of vital sign patterns are detected. The use of early warning scores using multiple inputs may prove useful in this arena.8 In a time when respiratory therapists are focusing more on intensive care patients, investing in technology and machine learning will be needed to reduce mortality in these patients at risk of respiratory compromise.
Footnotes
- Correspondence: David L Vines PhD MHS RRT FAARC, Rush University Medical Center, AAC 749, 600 S. Paulina St, Chicago, IL 60612. E-mail: david_vines{at}rush.edu
Dr Vines has disclosed relationships with Teleflex Medical, Fisher & Paykel Healthcare, and Ohio Medical.
See the Original Study on Page 1233
- Copyright © 2020 by Daedalus Enterprises







