Abstract
BACKGROUND: Improving FIO2 and reducing CO2 rebreathing (V̇ICO2) are the key means to improve the therapeutic efficacy of noninvasive ventilation (NIV). This study aimed to investigate the impact of interface design on FIO2 and V̇ICO2 during NIV.
METHODS: A simulated lung model was established to analyze 17 different interfaces. CO2 was injected into the outlet of the simulated lung, and the noninvasive ventilator was connected to the simulated lung to simulate the application of NIV in patients with COPD with hypercapnia. FIO2 and V̇ICO2 were calculated by mathematical integration of synchronously collected data pertaining to real-time pressure, flow, oxygen concentration, and CO2 concentration in the breathing circuit. Comparisons were performed between different types (nasal vs oronasal) and models of interfaces as well as between interfaces with different leak positions. Correlation of FIO2 and V̇ICO2 with inner volume and leakage, respectively, and the correlation between FIO2 and V̇ICO2 were analyzed.
RESULTS: FIO2 levels were significantly different with a nasal or an oronasal mask (0.45 ± 0.05% vs 0.41 ± 0.08%, respectively; P < .001). FIO2 levels associated with different models of interfaces varied significantly (all P < .001); V̇ICO2 did not differ significantly among the different interfaces (P = .19). Leak position significantly affected FIO2 and V̇ICO2 (all P < .001). Both inner volume and leakage significantly correlated with FIO2 (r = −0.23, P < .001; r = −0.08, P = .02). There was a significant correlation between FIO2 and V̇ICO2 (r = 0.43, P < .01); the general linear equation was y = 0.17 + 0.02x (r = 0.43, R2 = 0.19).
CONCLUSIONS: The design of the interface had a significant impact on FIO2 and V̇ICO2 during NIV. FIO2 and V̇ICO2 showed a significant positive correlation, although the effect size of correlation was moderate.
Introduction
Noninvasive ventilation (NIV) is a type of mechanical ventilation that entails the use of noninvasive interfaces such as a nasal mask or an oronasal mask to connect the patient to the ventilator.1 NIV is widely used to treat respiratory failure of diverse etiology. Appropriate use of NIV has been shown to reduce the need for endotracheal intubation, shorten the length of hospital stay, and decrease the mortality rate.2–4 Most noninvasive ventilators employ a single-limb breathing circuit; these require selective use of a passive exhalation port, which may cause CO2 rebreathing. Most home care noninvasive ventilators are not equipped with an air-oxygen blender to enable precise calibration of FIO2 and require extra oxygen injection; consequently, the actual FIO2 is often unknown.5 Improvement in FIO2 and reduction in the volume of CO2 rebreathing (V̇ICO2) is key to improve the therapeutic efficacy of NIV. However, several factors have been shown to affect FIO2 and V̇ICO2, such as the type of exhalation port, the position of the exhalation port in the breathing circuit, and leakage.6–10 Currently, most manufacturers use a mask design in which the exhalation port is integrated into the mask instead of the breathing circuit; in addition, various types and styles of interfaces have been designed to improve patient comfort. These different interfaces have distinct features with respect to appearance, position of exhalation port on the mask, leakage, and inner volume.11 However, the impact of these features on FIO2 level and V̇ICO2 during NIV is not well characterized. Therefore, we designed a bench study to investigate the influence of the design of different interfaces on FIO2 and V̇ICO2 during NIV.
Quick Look
Current Knowledge
Noninvasive ventilation (NIV) is a common treatment for respiratory failure. Improving FIO2 and reducing the volume of CO2 rebreathing (V̇ICO2) are the key means to improve the therapeutic efficacy of NIV. Different interfaces with distinct designs are used for NIV; however, the impact of these features on FIO2 and V̇ICO2 is not clear.
What This Paper Contributes to Our Knowledge
The designs of different interfaces with respect to shape, leak position, and inner volume had a significant impact on FIO2 and V̇ICO2. The design of the interface may affect the therapeutic efficacy of NIV.
Methods
Simulated Lung Model
The ASL 5000 respiratory simulation system (IngMar, Pittsburgh, Pennsylvania) is a breathing simulator that incorporates a digitally controlled piston inside a cylinder. The system allows investigators to preset the compliance, resistance, and inspiratory force, which facilitates the simulation of respiration in patients with various illnesses. We used the following parameters to simulate the respiratory mechanics of a patient with COPD, as described in previous studies:12,13 compliance, 60 mL/cm H2O; inspiratory resistance, 10 cm H2O/L/s; expiratory resistance, 15 cm H2O/L/s; maximum drop in inspiratory pressure, –8 cm H2O. To simulate the negative pressure created by respiratory muscles, 5% of the respiratory cycle time involved active inspiration, 3% involved end-inspiratory hold, and 15% was for the return of pressure to baseline. The frequency was set at 20 breaths/min.
NIV Simulation in a Patient With COPD With Hypercapnia
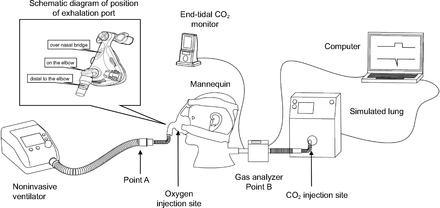
The simulated lung was connected to the back of a mannequin head through the breathing circuit to simulate spontaneous respiration (Figure 1). A noninvasive ventilator (Flexo, Curative Medical, Santa Clara, California) was connected to the front of the mannequin head through the ventilator-mannequin interface, and a single-limb breathing circuit was used to simulate NIV. To avoid unintended leaks, the inside of the mannequin head was sealed with glass glue, and the junction between the interface and the mannequin head was temporarily sealed with plasticine. The ventilator was set on the spontaneous breathing mode (S mode) with the pressure rising gradient set at 1 (implies that pressure was boosted at the fastest speed), inspiration sensitivity set at 1 (implies the highest inspiratory sensitivity), and expiration sensitivity set at 1 (implies the highest expiratory sensitivity). The inspiratory and expiratory pressures were set at 16 cm H2O and 4 cm H2O, respectively. Backup ventilation was disabled. To simulate oxygen supply during NIV, oxygen was injected into the breathing circuit at the rate of 5 L/min through the port on the interface or the site closest to the interface. CO2 was titrated into the outlet of the simulated lung using a micro-air flow controller (YJ-700CF, KongXing, Guilin, China) to simulate a patient with hypercapnia. The end-tidal CO2 was monitored using a vital sign monitor (NTID, Newtech Medical, Guangzhou, China) connected to the rear of the mannequin head (site B). The amount of injected CO2 gas was titrated to maintain an end-expiratory CO2 partial pressure of 80 mm Hg.
Schematic illustration of the experimental setup.
Measured Variables
For the evaluation of mask inner volume, all inlets connected to the ventilator and the exhalation port on the interface were sealed with tape. The sealed interfaces were filled with water, and the water subsequently transferred into a measuring cylinder (MasterScreen, Höchberg, Germany) to measure the volume. The reading of the cylinder was recorded by the same researcher for each mask tested. The average value from 3 independent measurements was used in the analysis.
With the noninvasive ventilator connected to the mannequin set in CPAP mode at 3 different pressure levels (ie, 4, 12, and 20 cm H2O) and the breathing circuit totally blocked at site B, a gas analyzer (VT-Plus, Fluke, Solon, Ohio) was connected to site A of the breathing circuit to measure the leakage with different interfaces at different pressure settings.
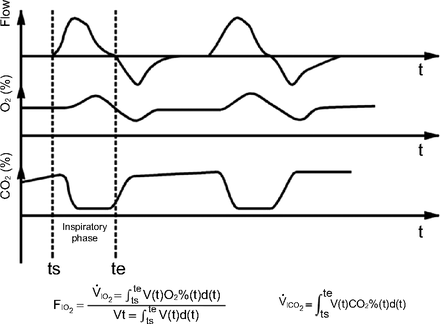
The measurement method was based on the principles of calculus and was similar to the post-processing of volumetric capnography and oxygen concentration measurements described previously.14 With an integrated flow and pressure sensor, CO2 sensor, and oxygen sensor, the custom-made gas analyzer used in our previous study14 located at site B of breathing circuit can synchronously collect real-time data on pressure, flow, oxygen concentration, and CO2 concentration in the breathing circuit at a sampling frequency of 30 Hz. The inspiratory phase was identified based on the flow waveform. At each sampling point of the inspiratory phase, the tidal volume was calculated based on the real-time gas flow, and the delivered volume of oxygen and CO2 was calculated by multiplying the tidal volume with the real-time oxygen concentration and CO2 concentration, respectively. The total delivered volume of oxygen and CO2 and the tidal volume were determined by mathematical integration of data at each sampling point. The equations for these calculations are shown in Figure 2. The delivered oxygen volume was divided by the tidal volume to determine FIO2. When the measured parameters were stable for 3 min, 10 cycles of FIO2 and V̇ICO2 were calculated under various experimental conditions.
Waveforms produced by the lung simulator to determine FIO2 and inspiratory volume of CO2 (V̇ICO2). ts = the time point of inspiratory start; te = the time point of inspiratory end.
Statistical Analysis
The distribution of all variables was assessed for normality using the 1-sample Kolmogorov-Smirnov test; the results were consistent with normal distribution (P < .05 for all). Data are presented as mean ± SD. One-way analysis of variance was applied to compare the effects of interface type (nasal mask vs oronasal mask), position of exhalation port, and different models of interface on FIO2 and V̇ICO2. Comparison between 2 pairs was performed using the least-significant difference test. Bivariate correlation analysis was used to assess the correlation of inner volume and leakage with FIO2 and V̇ICO2, respectively, as well as the correlation between FIO2 and V̇ICO2. Statistical analysis was performed using the statistical software SPSS 22.0 (IBM, Armonk, New York). Two-tailed P values < .05 indicated statistical significance.
Results
General Interface Characteristics
Nine models of oronasal mask and 8 models of nasal mask from 6 different manufacturers were tested in our study. The interfaces differed from one another in terms of inner volume, leakage, and position of the exhalation port (see Table 1). These interfaces can be categorized into 3 types according to the position of the exhalation port: in 4 interfaces, the leak port was located over the nasal bridge; in 9 interfaces, the leak port was located on the elbow of the interface; and in 4 interfaces, the leak port was located distal to the elbow on the interface (Fig. 1). Interfaces with 3 different positions of the leak port showed significantly different leakage (34.90 ± 10.90 L/min, 35.10 ± 11.19 L/min, and 30.95 ± 10.96 L/min, respectively; P < .001). Oronasal masks had significantly larger inner volume compared to nasal masks (218.76 ± 54.33 mL vs 108.18 ± 22.96 mL, P < .001). There was no significant difference between interfaces with different positions of the exhalation port for either the oronasal or the nasal masks (P = .59).
General Characteristics and the Associated Respiratory Variables of 17 Interfaces
The leakage of different interfaces showed a linear correlation with CPAP (r = 0.94, P < .001). The relation between CPAP (independent variable) and leakage (dependent variable) can be described by the linear equation y = 11.92 + 1.84x (R2 = 0.89). The leakage associated with different interfaces was significantly different at all CPAP levels (P < .001 for all). Oronasal masks showed significantly higher leakage compared to nasal masks (36.74 ± 11.46 mL vs 31.59 ± 10.18 mL; P < .001). There was a significant correlation between the leakage and the inner volume (r = 0.21, P < .001)
Factors Associated With FIO2 and V̇ICO2
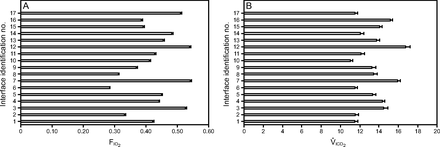
Significant differences in FIO2 levels were observed between different models of interfaces. Among the oronasal interfaces, the No. 6 interface was associated with the lowest FIO2 (0.28 ± 0%), whereas the No. 7 interface was associated with the highest FIO2 (0.54 ± 0%). Among the nasal interfaces, the No. 16 interface was associated with the lowest FIO2 (0.39 ± 0%), whereas the No. 12 interface was associated with the highest FIO2 (0.54 ± 0%) (Table 1, Fig. 3). The mean FIO2 achieved with nasal masks was significantly higher than that achieved with oronasal masks (0.45 ± 0.05% vs 0.41 ± 0.08%, respectively; P < .001). Interfaces with the exhalation port located over the nasal bridge, on the elbow, and distal to the elbow were associated with significantly different FIO2 levels (0.35 ± 0.07%, 0.46 ± 0.06%, and 0.39 ± 0.07%, respectively; P values for pair-wise comparison < .001).
Levels of FIO2 and inspiratory volume of CO2 (V̇ICO2) achieved with different interfaces.
Likewise, different models of interfaces showed significant differences with respect to V̇ICO2. Among the oronasal interfaces, the No. 1 interface was associated with the lowest V̇ICO2 (11.49 ± 0.33 mL), whereas the No. 7 interface was associated with the highest V̇ICO2 (15.91 ± 0.29 mL). Among the nasal interfaces, the No. 10 interface was associated with the lowest V̇ICO2 (11.01 ± 0.29 mL), whereas the No. 12 interface was associated with the highest V̇ICO2 (16.78 ± 0.46 mL) (Table 1, Fig. 3). Interfaces with the exhalation port located over the nasal bridge, on the elbow, and distal to the elbow were associated with significantly different V̇ICO2 levels (12.15 ± 0.87 mL, 13.39 ± 1.68 mL, and 14.38 ± 1.71 mL, respectively; P values for pair-wise comparison < .001). However, there was no significant difference between the oronasal masks and the nasal masks with respect to V̇ICO2 (13.23 ± 1.49 mL and 13.31 ± 1.91 mL, respectively; P = .19).
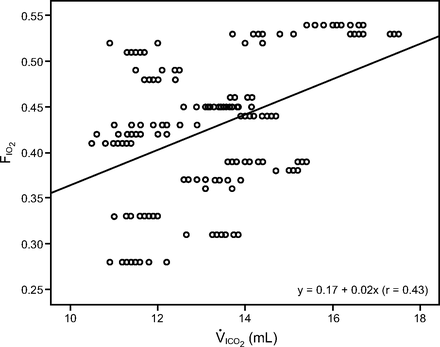
Both inner volume and leakage exhibited a significant negative correlation with FIO2 (r = −0.23, P < .001; r = −0.08, P = .02) but not with V̇ICO2 (r = –0.08, P = .29; r = –0.05, P = .50). FIO2 also showed a significant positive correlation with V̇ICO2; the inter-relationship can be described by the linear equation y = 0.17 + 0.02x (r = 0.43, A2 = 0.19), with V̇ICO2 as the independent variable x and FIO2 as the dependent variable y (Fig. 4).
Scatter plot showing the correlation between FIO2 and inspiratory volume of CO2 (V̇ICO2) with different interfaces.
Discussion
In this study, we investigated the influence of 17 different interfaces on FIO2 and V̇ICO2 through in vitro simulation of NIV treatment in COPD patients with hypercapnia. Different models of interfaces were associated with significantly different FIO2 and V̇ICO2 during NIV; the difference in the position of exhalation port was found to affect both FIO2 and V̇ICO2, while differences with respect to the inner volume and leakage affected FIO2 but not V̇ICO2. Further analysis revealed a significant positive correlation between FIO2 and V̇ICO2, although the effect size of the correlation was moderate.
Selection of the appropriate interface for a particular patient is the key to improve the therapeutic efficacy of NIV. Development of interfaces is largely focused on enhancement of comfort, prevention of leak, and customization of the wearing method through improvement in texture and geometric design15; however, the relationship between interface design and ventilator parameters (such as FIO2 and V̇ICO2) is not well characterized. The primary aim of NIV is to rest respiratory muscles, reduce CO2 retention, and improve oxygenation in patients with respiratory failure; therefore, FIO2 and V̇ICO2 are important parameters that should be considered during interface design.
Most noninvasive ventilators employ single-limb breathing circuits with a passive exhalation port. A proportion of the CO2-enriched exhaled gas is likely to remain within the breathing circuit and to be inhaled in the subsequent respiratory cycle, resulting in CO2 rebreathing.8,16 The discharge of CO2 during NIV largely relies on enhancement of alveolar ventilation and reduction of V̇ICO2, which may be affected by patient-associated factors (eg, basic CO2 level, spontaneous respiratory pattern), ventilator-associated parameters (eg, ventilation mode, inspiratory pressure, expiratory pressure, and trigger sensitivity), and interface-associated factors.6
In this study, we measured the leakage in a blocked breathing circuit at different CPAP levels and identified a significant linear correlation between these parameters. The measurement methods have been used for measuring the leakage of exhalation port in previous studies,14,17,18 although these methods may not capture the actual leakage. Indeed, the volume of leakage cannot be accurately measured because it changes synchronously with the periodic pressure fluctuations inside the breathing circuit during the respiratory cycle. We speculate that the current measurement method would not affect the conclusion regarding the linear correlation between leakage and pressure. However, this speculation needs be confirmed in a future study.
Theoretically, an interface with less dead space and greater expiratory leakage would better facilitate the elimination of CO2. However, small inner space may reduce the contact area, which makes it harder to stabilize the interface and increases patient discomfort. Likewise, an interface with large leakage may interfere with the ventilator trigger, resulting in patient-ventilator asynchrony, FIO2 reduction, and compromised therapeutic efficacy.19–21 All in all, inner volume and leakage need to be carefully calibrated to achieve a balance between these pros and cons and to maximize efficacy.
As shown in our study, oronasal masks have significantly larger inner volume and leakage compared to nasal masks; however, this did not translate into a significant difference in terms of V̇ICO2. The similar V̇ICO2 levels achieved with oronasal masks and nasal masks may be attributable to the contradictory effect of inner volume and leakage. There was a significant correlation between the leakage and inner volume. While oronasal masks showed large inner volume and leakage at the same time, the increase in V̇ICO2 caused by large inner volume seems to have been counteracted by the large leakage. Moreover, other interface-associated factors, such as geometric design and position of the exhalation port, may also act as confounding factors and influence V̇ICO2. As a limitation of the study design and methods, we failed to compare V̇ICO2 for interfaces with different inner volume or leakage parameters while simultaneously controlling for all other conditions. Consequently, we are unable to draw any definitive conclusions pertaining to the effect of inner volume or leakage on V̇ICO2.
Although most ventilator manufacturers integrate the exhalation port with the interface, the position of the exhalation port on the interface often varies. The position of the exhalation port can be broadly classified as over the nasal bridge, on the elbow of the interface, or distal to the elbow of the interface. In a previous study, interfaces with the exhalation port located on the interface (ie, over the nasal bridge) efficiently reduced V̇ICO2 compared to interfaces with the exhalation port located on the breathing circuit.8 No study has compared V̇ICO2 associated with an exhalation port located at different sites on the interface. Interfaces with the exhalation port in different positions exhibited significantly different leakage; however, we speculate that the difference in leakage was not the main cause of the influence of position on V̇ICO2. To our knowledge, this study is the first to indicate that having the exhalation port located over the nasal bridge may reduce V̇ICO2 better than having the exhalation port located at other sites on the interface.
In our previous study, FIO2 was significantly higher during NIV when the oxygen injection site was closer to the patient14; this contradicted findings reported by Schwartz et al21 and Waugh and De Kler.22 We speculate that the inconsistent findings may be associated with the different interfaces used in these studies. The oxygen injection site of the interfaces used in the studies by Schwartz et al17 and by Waugh and De Kler22 were located over the nasal bridge. The exhalation port of these interfaces were located downstream to the oxygen injection site and closer to the oxygen injection site; therefore, a part of the injected oxygen may have leaked directly through the exhalation port, resulting in reduced efficiency of oxygen supply. However, in our previous study, the exhalation port was located on the elbow of the interface while the oxygen injection was on the lateral wall of the interface.14 When oxygen is injected into the interface through the lateral wall, the exhalation port is located upstream to the oxygen injection site; this prevents excessive leakage of oxygen through the exhalation port. Consistent with this hypothesis, our results also suggest that the position of the exhalation port may significantly affect FIO2. The lowest FIO2 was detected with interfaces in which the exhalation port was located over the nasal bridge.
Currently there are no strict recommendations pertaining to the oxygen injection site; however, most manufacturers tend to place the site at the outlet of the ventilator. To some extent, the choice of oxygen injection site is based on usage habit and the local situation. In China, home care ventilators are commonly used in hospitals and the oxygen injection site is often located on the mask. For patients with hypoxemia, the location of the oxygen injection site on the interface may facilitate higher FIO2 and help improve the oxygen supply. Therefore, we only used the site located on the mask in this study. Although we believe that the oxygen injection site may also influence V̇ICO2, the magnitude of the influence is still unclear. In addition, the focus of this study was on the influence of different interfaces on V̇ICO2 rather than on the influence of oxygen injection site.
The gas within the cavity of the interface is a mixture of gas from 3 different origins: exhaled gas enriched with CO2, injected oxygen, and fresh air. V̇ICO2 and FIO2 depend on the fractions of these components during the inspiratory phase. Except for the oxygen that is supplied at a constant rate, the inflow of the other 2 components and the outflow of all 3 components vary with time; this results in dynamic changes in the fractions of CO2 and O2. In addition, the interface-associated factors (including shape, inner volume, leakage, and position of exhalation port) may have a complex effect on the mixture and discharge of the 3 gases. Theoretically, the weaker the ability of the interface to remove dead-space gas, the lesser the elimination of CO2 or O2, and, consequently, the higher V̇ICO2 and FIO2 will be. This explains the positive correlation observed between V̇ICO2 and FIO2 in our study.
A major limitation of this study was the in vitro study design. Therefore, the study setting was not fully representative of real-life NIV treatment in patients. The parameters of the simulated lung were not completely consistent with the physiological conditions and only simulated a part of the respiratory mechanics of patients with COPD. In addition, the simulated lung does not consume the inhaled oxygen; previous studies have reported that FIO2 measured in vitro is slightly higher than that measured in clinical trials.23 Furthermore, we simulated hypercapnia through continuous injection of CO2 into the breathing circuit, as described in previous reports.8,24 Whether V̇ICO2 measured in this way represents the actual situation in patients is yet to be verified. Moreover, some studies have reported that the respiratory center only responds to > 0.1% change in CO2 concentration due to rebreathing.6,25 Thus, our in vitro study can only demonstrate the potential effects of different interfaces on FIO2 and V̇ICO2. Further clinical studies are required to verify the clinical importance of our findings.
All interface-associated factors (eg, geometric design, position of the exhalation port, inner volume, and leakage) may have a potential confounding effect on FIO2 and V̇ICO2. It is difficult to clearly delineate the mask features associated with high FIO2 or low V̇ICO2. In addition, the purpose of this study was not simply to compare the advantages and disadvantages of different interfaces in term of FIO2 and V̇ICO2. Indeed, in most cases, the effect of the interface on FIO2 and V̇ICO2 can be counterbalanced by calibrating the ventilator parameters or oxygen flow. However, knowledge of the effect of the interface on FIO2 and V̇ICO2 may help clinicians in accurately evaluating disease severity and help improve therapeutic efficacy. In addition, insights from this study may help design better interfaces.
Conclusions
Our results indicate that differences between interfaces in terms of inner volume, leakage, and different positions of the exhalation port may result in variance in V̇ICO2. The position of the exhalation port can influence both FIO2 and V̇ICO2, whereas inner volume and leakage affect only FIO2. FIO2 and V̇ICO2 showed a positive correlation.
Footnotes
- Correspondence: Bing Dai MD, Department of Respiratory and Critical Care Medicine, First Affiliated Hospital of China Medical University, No.155, Nanjing North St, Heping District, Shenyang 110001, China. E-mail: dai6206856{at}163.com
The authors have disclosed no conflicts of interest.
- Copyright © 2021 by Daedalus Enterprises