Abstract
BACKGROUND: Oxygen administration is recommended for patients with hypoxemia to achieve a target 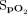 range. Strategies to achieve this in clinical practice are suboptimal. We investigated automatic oxygen titration using a novel nasal high-flow device with closed-loop oxygen control. The objective of this proof-of-concept study was to determine whether closed-loop control was able to respond to desaturation and subsequent recovery in a controlled laboratory-based environment.
range. Strategies to achieve this in clinical practice are suboptimal. We investigated automatic oxygen titration using a novel nasal high-flow device with closed-loop oxygen control. The objective of this proof-of-concept study was to determine whether closed-loop control was able to respond to desaturation and subsequent recovery in a controlled laboratory-based environment.
METHODS: We conducted a single-blind randomized crossover trial in adults with chronic respiratory disease who had a resting 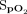 ≥ 92% and desaturated to < 90% during a 6-min walk test (6MWT). Nasal high-flow was administered during a 6MWT and a subsequent 10-min rest period with either room air, a fixed concentration of 28% oxygen, or oxygen titrated automatically using closed-loop control.
≥ 92% and desaturated to < 90% during a 6-min walk test (6MWT). Nasal high-flow was administered during a 6MWT and a subsequent 10-min rest period with either room air, a fixed concentration of 28% oxygen, or oxygen titrated automatically using closed-loop control.
RESULTS: The study involved 42 subjects. Closed-loop control maintained 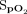 within the target range of 92–96% for a mean (SD) duration of 54.4 ± 30.1% of the 6MWT and 67.3 ± 26.8% of the recovery period. The proportion of time spent with an
within the target range of 92–96% for a mean (SD) duration of 54.4 ± 30.1% of the 6MWT and 67.3 ± 26.8% of the recovery period. The proportion of time spent with an 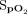 in the target range during the 6MWT was significantly greater for closed-loop control compared to room air, with a difference of 26.0% (95% CI 17.7–34.2, P < .001); this proportion of time was not significantly different compared to the fixed concentration of 28% oxygen, with a difference of –8.2% (95% CI –16.5 to 0.1, P = .052). The proportion of time spent in the target range during the rest period was significantly greater compared to 28% oxygen, with a difference of 19.3% (95% CI 8.9–29.7, P < .001); this proportion of time was not significantly different compared to room air, with a difference of –9.3% (95% CI –19.7 to 1.0, P = .08).
in the target range during the 6MWT was significantly greater for closed-loop control compared to room air, with a difference of 26.0% (95% CI 17.7–34.2, P < .001); this proportion of time was not significantly different compared to the fixed concentration of 28% oxygen, with a difference of –8.2% (95% CI –16.5 to 0.1, P = .052). The proportion of time spent in the target range during the rest period was significantly greater compared to 28% oxygen, with a difference of 19.3% (95% CI 8.9–29.7, P < .001); this proportion of time was not significantly different compared to room air, with a difference of –9.3% (95% CI –19.7 to 1.0, P = .08).
CONCLUSIONS: This study provides proof-of-concept evidence that the novel nasal high-flow device with closed-loop control can respond to changes in  outside a target saturation range using a model of exercise-induced desaturation and subsequent recovery.
outside a target saturation range using a model of exercise-induced desaturation and subsequent recovery.
Introduction
Oxygen is commonly administered in the acute care setting,1 with the aim of correcting hypoxemia and improv-ing tissue oxygenation. While inadequate treatment of hypoxemia should be avoided, excessive administration of oxygen and hyperoxemia also lead to harm in a number of disease states.2-4 International guidelines therefore recommend titration of oxygen to achieve peripheral 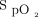 within a specific range.5,6 Manual oxygen titration is utilized in clinical practice, but this is both challenging and time-consuming7 and it may contribute to suboptimal oxygen administration due to changes in a patient’s condition and activity over time.8
within a specific range.5,6 Manual oxygen titration is utilized in clinical practice, but this is both challenging and time-consuming7 and it may contribute to suboptimal oxygen administration due to changes in a patient’s condition and activity over time.8
Oxygen titration using a closed-loop control system allows continuous automated adjustment of a delivered oxygen concentration to achieve an 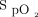 within a desired range. In laboratory-based exercise tests, several studies have reported a greater proportion of time spent within a target
within a desired range. In laboratory-based exercise tests, several studies have reported a greater proportion of time spent within a target 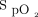 range using closed-loop oxygen control in comparison to delivery of a fixed concentration of oxygen in adults with chronic stable lung disease.9-12 Similar findings have also been reported when compared to manual oxygen titration in nonventilated adult subjects with acute illnesses.13-16
range using closed-loop oxygen control in comparison to delivery of a fixed concentration of oxygen in adults with chronic stable lung disease.9-12 Similar findings have also been reported when compared to manual oxygen titration in nonventilated adult subjects with acute illnesses.13-16
To date, these studies have relied upon conventional low-flow oxygen therapy.13-16 Nasal high-flow (HFNC) oxygen therapy involves delivery of heated humidified gas at high flow, which has a number of beneficial effects including generation of PEEP,17,18 reduction in work of breathing,18 delivery of a more predictable inspired oxygen concentration,19,20 and dead space washout.21 HFNC has also been shown to improve clinical outcomes compared to conventional oxygen therapy in acute hypoxaemic respiratory failure.22,23 The use of closed-loop control with HFNC is therefore an appealing method of oxygen delivery.
This is the first study to evaluate a HFNC device with closed-loop oxygen control (Airvo 3, Fisher and Paykel Healthcare, Auckland, New Zealand). The device consists of a flow generator, a humidifier, and a blender that mixes entrained air with oxygen. The device can automatically titrate the delivered oxygen concentration to achieve a user-set target 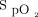 range. The adjustment in delivered oxygen concentration is managed by an adaptive controller. The rate of adjustment in oxygen every second is dependent on the magnitude of deviation of the current
range. The adjustment in delivered oxygen concentration is managed by an adaptive controller. The rate of adjustment in oxygen every second is dependent on the magnitude of deviation of the current 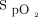 value from the target value, as well as the direction of the
value from the target value, as well as the direction of the 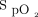 trend. The magnitude of adjustment is also affected by how the patient has previously responded to changes in oxygen concentration.
trend. The magnitude of adjustment is also affected by how the patient has previously responded to changes in oxygen concentration.
This proof-of-concept study was designed to evaluate closed-loop oxygen control using the novel HFNC device in response to desaturation and recovery in subjects with chronic stable lung disease during a 6-min walk test (6MWT) and a rest period. This study design was chosen to provide a safe model of desaturation against which HFNC with closed-loop control could be tested in a controlled environment; this study was not intended to assess the clinical utility of the device as a potential means of delivering ambulatory oxygen therapy.
Device responsiveness was tested in terms of its ability to maintain 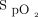 within a specified target range during subjects’ exertion and recovery. This was compared with the delivery of room air at high flow and a fixed concentration of oxygen at high flow. This comparison was chosen to provide a reference against which closed-loop control could be assessed; we hypothesized that closed-loop control would maintain
within a specified target range during subjects’ exertion and recovery. This was compared with the delivery of room air at high flow and a fixed concentration of oxygen at high flow. This comparison was chosen to provide a reference against which closed-loop control could be assessed; we hypothesized that closed-loop control would maintain 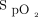 within the target range for a greater proportion of time than the other interventions.
within the target range for a greater proportion of time than the other interventions.
QUICK LOOK
Current Knowledge
In clinical practice, manual oxygen titration to achieve a desired target peripheral 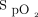 range is challenging. Closed-loop oxygen control has been shown to increase the proportion of time spent with
range is challenging. Closed-loop oxygen control has been shown to increase the proportion of time spent with 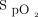 in a desired target range in both laboratory-based exercise tests and in patients with acute illness.
in a desired target range in both laboratory-based exercise tests and in patients with acute illness.
What This Paper Contributes to Our Knowledge
HFNC with was compared to HFNC with room air and HFNC with a fixed concentration of 28% oxygen during a 6-min walk test (6MWT) and a 10-min recovery period in subjects with stable chronic lung disease. closed-loop control resulted in a greater proportion of time spent with  in the target range during the 6MWT compared to room air, but was not significantly different from 28% oxygen. Conversely, closed-loop control resulted in a greater proportion of time spent with
in the target range during the 6MWT compared to room air, but was not significantly different from 28% oxygen. Conversely, closed-loop control resulted in a greater proportion of time spent with  in the target range during the rest period compared to 28% oxygen, but was not significantly different from room air.
in the target range during the rest period compared to 28% oxygen, but was not significantly different from room air.
Methods
Study Design
This was a single-blind, randomized, 3-way crossover trial performed at the Medical Research Institute of New Zealand. Ethical approval was obtained from the Northern B Health and Disability Ethics Committee. The study was run in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. This trial was registered with the Australian and New Zealand Clinical Trials Registry (ACTRN12618001144202).
Subjects
Subjects were adults with stable chronic respiratory disease who had a resting 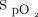 ≥ 92% and desaturated to < 90% during a 6MWT. Exclusion criteria included presence of any absolute or relative contraindications (at investigator discretion) to performing a 6MWT as per American Thoracic Society/European Respiratory Society guidelines24 or recent (ie, within 4 weeks) exacerbation of their respiratory condition. All subjects gave written informed consent.
≥ 92% and desaturated to < 90% during a 6MWT. Exclusion criteria included presence of any absolute or relative contraindications (at investigator discretion) to performing a 6MWT as per American Thoracic Society/European Respiratory Society guidelines24 or recent (ie, within 4 weeks) exacerbation of their respiratory condition. All subjects gave written informed consent.
Randomization
Subjects performed 3 6MWTs in a random order. The randomization code was generated by the study statistician using a computer-generated sequence. Allocation was concealed by the REDCap electronic case report form and was released to investigators at time of randomization.
Procedures
Subjects attended an initial screening visit to determine eligibility. At this visit, 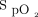 , FEV1, and forced vital capacity were measured. A 6MWT was performed with the subject breathing room air.
, FEV1, and forced vital capacity were measured. A 6MWT was performed with the subject breathing room air. 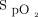 was measured continuously using a disposable adhesive finger sensor and pulse oximeter (sat 801+, Bitmos, Düsseldorf, Germany), which was carried by an investigator alongside the subject. Desaturation to < 90% was determined by analyzing the pulse oximeter data (satView software 1.1.9, Bitmos).
was measured continuously using a disposable adhesive finger sensor and pulse oximeter (sat 801+, Bitmos, Düsseldorf, Germany), which was carried by an investigator alongside the subject. Desaturation to < 90% was determined by analyzing the pulse oximeter data (satView software 1.1.9, Bitmos).
Eligible subjects returned to the Medical Research Institute of New Zealand on a different day to undertake 3 6MWTs using the HFNC device set to deliver room air (HFNC room air), a fixed concentration of 28% oxygen (HFNC O2), or closed-loop oxygen control (HFNC-closed loop) to achieve a target 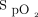 of 92–96%. The fixed concentration of 28% oxygen was chosen as an approximate equivalent to 2 L/min of low-flow oxygen, which is a typical flow used for ambulatory oxygen and was therefore expected to maintain
of 92–96%. The fixed concentration of 28% oxygen was chosen as an approximate equivalent to 2 L/min of low-flow oxygen, which is a typical flow used for ambulatory oxygen and was therefore expected to maintain  close to the target range during a 6MWT. A flow of 35 L/min was used for all interventions. During closed-loop control, the delivered oxygen concentration could be titrated by the device between 21% and 55%. The
close to the target range during a 6MWT. A flow of 35 L/min was used for all interventions. During closed-loop control, the delivered oxygen concentration could be titrated by the device between 21% and 55%. The 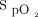 target was chosen to represent normoxemia for subjects, all of whom had resting
target was chosen to represent normoxemia for subjects, all of whom had resting  ≥ 92%. During each 6MWT, an investigator pushed a mobile stand alongside the subject, which supported the HFNC device and oxygen cylinder. The warmed, humidified mixture of air and oxygen was delivered using an AirSpiral heated breathing tube (Fisher & Paykel Healthcare) and Optiflow+ nasal cannula (Fisher & Paykel Healthcare). A disposable adhesive finger sensor was placed on each hand, with one connected to the HFNC device and the other connected to the independent pulse oximeter. The independent pulse oximeter was used to corroborate the
≥ 92%. During each 6MWT, an investigator pushed a mobile stand alongside the subject, which supported the HFNC device and oxygen cylinder. The warmed, humidified mixture of air and oxygen was delivered using an AirSpiral heated breathing tube (Fisher & Paykel Healthcare) and Optiflow+ nasal cannula (Fisher & Paykel Healthcare). A disposable adhesive finger sensor was placed on each hand, with one connected to the HFNC device and the other connected to the independent pulse oximeter. The independent pulse oximeter was used to corroborate the 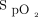 data from the HFNC device and otherwise did not provide data for analysis. All 6MWTs were stopped in the event of desaturation < 80% as a safety measure. Subjects were not aware of the device settings used for each 6MWT; investigators were not blinded to the allocated order of interventions.
data from the HFNC device and otherwise did not provide data for analysis. All 6MWTs were stopped in the event of desaturation < 80% as a safety measure. Subjects were not aware of the device settings used for each 6MWT; investigators were not blinded to the allocated order of interventions.
Before and after each 6MWT, an investigator obtained a fatigue and dyspnea score from the subjects using the modified Borg Scale.25 At the end of the 6MWT, each subject was transferred via wheelchair from the corridor to the seated recovery area where they underwent continued monitoring for a 10- min seated recovery period. During this time, each subject continued to use the device with the same settings as the preceding 6MWT. After the 10-min seated recovery period and prior to commencing the next 6MWT, there was a 1-h rest period, during which the subject breathed ambient air without the device.
Outcomes
The primary outcome of the study was the proportion of time spent during the 6MWT with  in the target range of 92–96%. If the walk test was stopped before 6 min, the proportion of the completed duration of the 6MWT in target range was used. Secondary outcomes included proportions of time spent within prespecified
in the target range of 92–96%. If the walk test was stopped before 6 min, the proportion of the completed duration of the 6MWT in target range was used. Secondary outcomes included proportions of time spent within prespecified 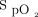 thresholds (ie, < 90%, < 92%, > 96%, > 98%), heart rate, delivered oxygen concentration, the proportion of time spent with
thresholds (ie, < 90%, < 92%, > 96%, > 98%), heart rate, delivered oxygen concentration, the proportion of time spent with 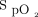 at 92–96% during the 10-min recovery period, and the limits of agreement of
at 92–96% during the 10-min recovery period, and the limits of agreement of  measured by the HFNC device and an independent pulse oximeter. Other outcomes were total distance walked and change in modified Borg scores. Data recorded by the HFNC device were used for all outcomes relating to
measured by the HFNC device and an independent pulse oximeter. Other outcomes were total distance walked and change in modified Borg scores. Data recorded by the HFNC device were used for all outcomes relating to 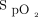 , heart rate, and delivered oxygen concentration.
, heart rate, and delivered oxygen concentration.
Statistical Analysis
Based on a previous study, which used an automatic oxygen titration system during walking in subjects with COPD12 and a paired standard deviation (SD) of 33, a sample size of 42 had at least 90% power at an alpha of 5% to detect a 20% difference in the proportion of time spent within the target range.
The statistical analysis was an intention-to-treat superiority analysis. The 2 prespecified comparisons were between closed-loop control and room air, and between closed-loop control and 28% oxygen. A mixed linear model was used for the primary outcome variable, with fixed effects for baseline resting  , randomized order of treatment, and oxygen delivery, and random effects for each subject with an unstructured variance-covariance matrix. The statistical methods used for the other outcome variables is provided in the online appendix (see the supplementary materials at http://www.rcjournal.com). For the locally estimated scatter plot smoother (LOESS) plots, a smoothing parameter of 0.5 was used, together with approximate degrees of freedom and a 2-sided alpha of 5% to give a 95% CI. For the larger data set of 600 recordings per subject over 10 min every second measurement was used to manage the algorithm to generate the plots and confidence intervals. SAS 9.4 (SAS Institute, Cary, North Carolina) was used for analyses.
, randomized order of treatment, and oxygen delivery, and random effects for each subject with an unstructured variance-covariance matrix. The statistical methods used for the other outcome variables is provided in the online appendix (see the supplementary materials at http://www.rcjournal.com). For the locally estimated scatter plot smoother (LOESS) plots, a smoothing parameter of 0.5 was used, together with approximate degrees of freedom and a 2-sided alpha of 5% to give a 95% CI. For the larger data set of 600 recordings per subject over 10 min every second measurement was used to manage the algorithm to generate the plots and confidence intervals. SAS 9.4 (SAS Institute, Cary, North Carolina) was used for analyses.
Results
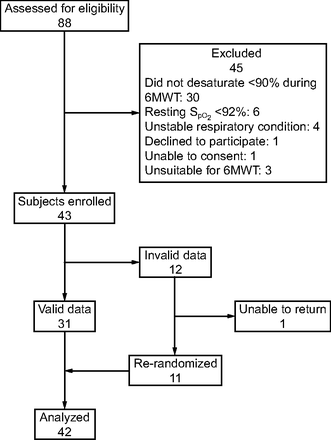
Subjects were recruited to the study between September 12, 2018, and June 12, 2019. A total of 88 patients were assessed for eligibility, of whom 45 were excluded (Fig. 1). After the first 12 subjects were randomized, we detected a device fault causing a delay in automatic oxygen titration, which invalidated the data collected up to that point. After correction of the device fault, 11 of the initial subjects were re-randomized and an additional 31 subjects were recruited to make a total of 42 randomized subjects with valid data in the analysis. The invalid data collected from the 12 subjects were discarded.
Flow chart. 6MWT = 6-min walk test.
Baseline subject characteristics are summarized in Table 1. The majority of subjects were elderly (mean age 71 y) and male (n = 26). The most common respiratory diagnosis was COPD (n = 36), with the remainder of subjects having interstitial lung disease (n = 5) or bronchiectasis (n = 1). Mean ± SD resting 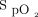 was 94.1 ± 1.5%, and the mean ± SD distance achieved during the screening 6MWT was 308.8 ± 128.7 m with a mean ± SD minimum
was 94.1 ± 1.5%, and the mean ± SD distance achieved during the screening 6MWT was 308.8 ± 128.7 m with a mean ± SD minimum 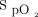 of 83.7 ± 4.1%. The 6MWT was stopped early for desaturation < 80% for 2, 8, and 3 6MWTs for the HFNC closed loop, HFNC room air, and HFNC fixed O2 interventions, respectively. No subjects were prescribed ambulatory oxygen therapy.
of 83.7 ± 4.1%. The 6MWT was stopped early for desaturation < 80% for 2, 8, and 3 6MWTs for the HFNC closed loop, HFNC room air, and HFNC fixed O2 interventions, respectively. No subjects were prescribed ambulatory oxygen therapy.
Baseline Subject Characteristics
Using closed-loop control,  was maintained within target range for a mean ± SD duration of 54.4 ± 30.1% of the 6MWT. Closed-loop control resulted in a significantly greater proportion of time spent with
was maintained within target range for a mean ± SD duration of 54.4 ± 30.1% of the 6MWT. Closed-loop control resulted in a significantly greater proportion of time spent with 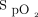 in the target range compared to room air: HFNC closed loop minus HFNC room air = 26.0% (95% CI 17.7–34.2, P < .001) (Table 2, Table 3, Figure 2). There was no significant difference in the proportion of time spent in target range during the 6MWT with closed-loop control compared to 28% oxygen: HFNC closed loop minus HFNC fixed O2 = –8.2% (–16.5 to 0.1, P = .052). The estimates of differences were similar in a sensitivity analysis with FEV1% and
in the target range compared to room air: HFNC closed loop minus HFNC room air = 26.0% (95% CI 17.7–34.2, P < .001) (Table 2, Table 3, Figure 2). There was no significant difference in the proportion of time spent in target range during the 6MWT with closed-loop control compared to 28% oxygen: HFNC closed loop minus HFNC fixed O2 = –8.2% (–16.5 to 0.1, P = .052). The estimates of differences were similar in a sensitivity analysis with FEV1% and 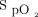 nadir during the screening 6MWT as covariates (see the supplementary materials at http://www.rcjournal.com). The pattern of
nadir during the screening 6MWT as covariates (see the supplementary materials at http://www.rcjournal.com). The pattern of 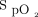 response to the interventions during the 6MWT and recovery period is shown in Figure 3. The pattern of change in the delivered oxygen concentration is shown in Figure 4. Corresponding plots demonstrating individual subject
response to the interventions during the 6MWT and recovery period is shown in Figure 3. The pattern of change in the delivered oxygen concentration is shown in Figure 4. Corresponding plots demonstrating individual subject  responses and median
responses and median 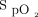 for the 6MWT and 10-min recovery period, the inter-subject variability in
for the 6MWT and 10-min recovery period, the inter-subject variability in 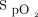 at 1-min intervals during the 6MWT and during the 10-min recovery period, and the median delivered oxygen concentration for the 6MWT and the 10-min recovery period are shown in the supplementary materials (available at http://www.rcjournal.com). As a result of inter-subject variability in individual
at 1-min intervals during the 6MWT and during the 10-min recovery period, and the median delivered oxygen concentration for the 6MWT and the 10-min recovery period are shown in the supplementary materials (available at http://www.rcjournal.com). As a result of inter-subject variability in individual  measurements, the median
measurements, the median 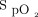 plots more accurately reflect the time spent with
plots more accurately reflect the time spent with  in the target range compared to the LOESS plots. The delivered oxygen concentration during the 6MWT varied from 21% to 55% with the closed-loop control intervention.
in the target range compared to the LOESS plots. The delivered oxygen concentration during the 6MWT varied from 21% to 55% with the closed-loop control intervention.
Outcome Measures for Randomized Treatments During 6-min Walk Test
Differences in Outcome Measures Between Treatments
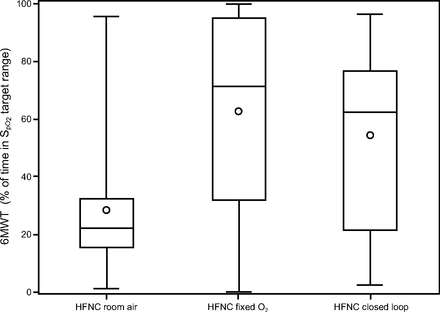
Boxplots display percentage of time spent with 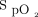 in target range (92–96%) during the 6MWT, according to randomized treatment. Whiskers denote minimum and maximum values, horizontal lines are the 25th, 50th (median) and 75th percentiles, and points show the mean. 6MWT = 6-min walk test.
in target range (92–96%) during the 6MWT, according to randomized treatment. Whiskers denote minimum and maximum values, horizontal lines are the 25th, 50th (median) and 75th percentiles, and points show the mean. 6MWT = 6-min walk test.
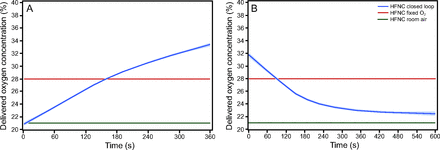
A: Locally estimated scatter plot smoother (LOESS) plot of 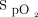 during the 6MWT. B: LOESS plot of
during the 6MWT. B: LOESS plot of 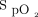 during the 10-min recovery period. 6MWT = 6-min walk test; HFNC = high-flow nasal cannula.
during the 10-min recovery period. 6MWT = 6-min walk test; HFNC = high-flow nasal cannula.
A: Locally estimated scatter plot smoother (LOESS) of delivered oxygen concentration during the 6MWT. B: LOESS plot of delivered oxygen concentration during the 10-min recovery period. 6MWT = 6-min walk test; HFNC = high-flow nasal cannula.
The use of closed-loop control resulted in a reduction in proportion of time spent with 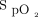 < 92% and < 90% during the 6MWT when compared to room air but not when compared to 28% oxygen (Table 3). The mean and minimum
< 92% and < 90% during the 6MWT when compared to room air but not when compared to 28% oxygen (Table 3). The mean and minimum 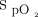 measurements during the 6MWT were higher for closed-loop control compared to room air: HFNC closed loop minus HFNC room air = 2.37% (95% CI 1.73–3.01, P < .001) and 2.71% (95% CI 1.56–3.86, P < .001), respectively. These estimates were lower compared to 28% oxygen: HFNC closed loop minus HFNC fixed O2 = –0.65% (95% CI –1.30 to –0.01, P = .047) and –2.12% (95% CI –3.27 to –0.97, P < .001), respectively.
measurements during the 6MWT were higher for closed-loop control compared to room air: HFNC closed loop minus HFNC room air = 2.37% (95% CI 1.73–3.01, P < .001) and 2.71% (95% CI 1.56–3.86, P < .001), respectively. These estimates were lower compared to 28% oxygen: HFNC closed loop minus HFNC fixed O2 = –0.65% (95% CI –1.30 to –0.01, P = .047) and –2.12% (95% CI –3.27 to –0.97, P < .001), respectively.
Using closed-loop control, 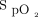 was maintained within target range for a mean ± SD duration of 67.3 ± 26.8% of the 10-min recovery period (Table 4). There was no significant difference in the proportion of time spent with
was maintained within target range for a mean ± SD duration of 67.3 ± 26.8% of the 10-min recovery period (Table 4). There was no significant difference in the proportion of time spent with 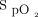 in the target range during the 10-min recovery period between closed-loop control and room air; HFNC closed loop minus HFNC room air = –9.3% (95% CI –19.7 to 1.0, P = .08). However, closed-loop control resulted in a significantly greater proportion of time spent in range compared to 28% oxygen: HFNC closed loop minus HFNC fixed O2 = 19.3% (95% CI 8.9–29.7, P < .001) (Table 3). There was no evidence of differences in any of the other secondary outcome measures (Table 3), nor was there evidence of bias between the
in the target range during the 10-min recovery period between closed-loop control and room air; HFNC closed loop minus HFNC room air = –9.3% (95% CI –19.7 to 1.0, P = .08). However, closed-loop control resulted in a significantly greater proportion of time spent in range compared to 28% oxygen: HFNC closed loop minus HFNC fixed O2 = 19.3% (95% CI 8.9–29.7, P < .001) (Table 3). There was no evidence of differences in any of the other secondary outcome measures (Table 3), nor was there evidence of bias between the 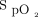 as measured with the HFNC device and with the independent pulse oximeter (see the supplementary materials at http://www.rcjournal.com).
as measured with the HFNC device and with the independent pulse oximeter (see the supplementary materials at http://www.rcjournal.com).
Outcome Measures for Randomized Treatments During 10-min Recovery Period
The use of closed-loop control resulted in an increase in the mean distance walked when compared to room air: HFNC closed loop minus HFNC room air = 32.2 m (95% CI 15.3–49.1, P < .001), but not when compared to 28% oxygen: HFNC closed loop minus HFNC fixed O2 = 4.4 m (95% CI –12.5 to 21.3, P = .61). There was no difference between treatments in change in modified Borg fatigue and dyspnea scores (Table 3). No serious adverse events were reported.
Discussion
The results of this study indicate that the novel HFNC device with closed-loop oxygen control was able to respond to desaturation and recovery in subjects with chronic lung disease during a 6MWT and a rest period. With reference to the comparator interventions, during the 6MWT closed-loop control was able to maintain 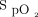 within the target range more effectively than delivery of room air and did not differ significantly from delivery of 28% oxygen. In the recovery period, closed-loop control was able to maintain
within the target range more effectively than delivery of room air and did not differ significantly from delivery of 28% oxygen. In the recovery period, closed-loop control was able to maintain  within the target range more effectively than 28% oxygen and did not differ significantly from delivery of room air.
within the target range more effectively than 28% oxygen and did not differ significantly from delivery of room air.
The 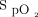 response to the 3 interventions over the time course of the 6MWT and recovery period are well illustrated in the LOESS plots shown in Figure 3. There was separation of
response to the 3 interventions over the time course of the 6MWT and recovery period are well illustrated in the LOESS plots shown in Figure 3. There was separation of 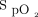 between closed-loop control and room air after approximately 1 min, with the
between closed-loop control and room air after approximately 1 min, with the 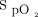 during closed-loop control reaching a nadir after 2 min before increasing to the target range at the end of the 6MWT. This is in contrast to the pattern observed with 28% oxygen, which resulted in a delayed decrease in
during closed-loop control reaching a nadir after 2 min before increasing to the target range at the end of the 6MWT. This is in contrast to the pattern observed with 28% oxygen, which resulted in a delayed decrease in 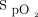 , a higher nadir, and no subsequent increase in
, a higher nadir, and no subsequent increase in  as the 6MWT continued. The time-lag effect observed with closed-loop control is likely to result from both the mechanism responding to a drop in
as the 6MWT continued. The time-lag effect observed with closed-loop control is likely to result from both the mechanism responding to a drop in 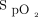 by increasing the delivered oxygen concentration and the natural time lag between the pulmonary and peripheral circulation. In contrast, the fixed concentration of 28% oxygen prevented this early desaturation but was unable to recover
by increasing the delivered oxygen concentration and the natural time lag between the pulmonary and peripheral circulation. In contrast, the fixed concentration of 28% oxygen prevented this early desaturation but was unable to recover 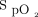 in the face of sustained desaturation in the latter part of the 6MWT.
in the face of sustained desaturation in the latter part of the 6MWT.
There was separation of the  between closed-loop control and 28% oxygen, which in both cases was associated with
between closed-loop control and 28% oxygen, which in both cases was associated with 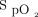 above the upper limit of 96% during the early part of the recovery period.
above the upper limit of 96% during the early part of the recovery period. 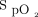 returned to the target range after approximately 4 min in response to closed-loop control, but not until the end of the 10-min recovery period in response to 28% oxygen. Again, a time-lag effect was observed with closed-loop control that required a brief period of overoxygenation for the mechanism to respond and reduce the delivered oxygen concentration. In contrast, the fixed concentration of 28% oxygen resulted in overoxygenation for the majority of the recovery period.
returned to the target range after approximately 4 min in response to closed-loop control, but not until the end of the 10-min recovery period in response to 28% oxygen. Again, a time-lag effect was observed with closed-loop control that required a brief period of overoxygenation for the mechanism to respond and reduce the delivered oxygen concentration. In contrast, the fixed concentration of 28% oxygen resulted in overoxygenation for the majority of the recovery period.
The nature of a closed-loop control mechanism requires a variable to deviate from a desired value for the controller to respond and bring the variable back toward the desired value or range. The speed of adjustment affected by the controller is balanced between the need to promptly restore the variable back toward the desired range and the need to avoid overcorrection. This will necessarily result in a time lag and a period of time spent with the variable outside of the desired range. An upward trend in  was observed with closed-loop control in the latter part of the 6MWT, and a downward trend was noted during the 10-min recovery period, bringing
was observed with closed-loop control in the latter part of the 6MWT, and a downward trend was noted during the 10-min recovery period, bringing 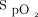 back toward the target range in both circumstances. The capability of the closed-loop system to respond appropriately to desaturation provoked by exercise was shown by the variation in delivered oxygen concentration, with a range of 21–55% within the 6-min period of the exercise test. Considering these factors, we propose that the results of this study provide proof-of-concept evidence that the closed-loop control system is able to respond to changes in
back toward the target range in both circumstances. The capability of the closed-loop system to respond appropriately to desaturation provoked by exercise was shown by the variation in delivered oxygen concentration, with a range of 21–55% within the 6-min period of the exercise test. Considering these factors, we propose that the results of this study provide proof-of-concept evidence that the closed-loop control system is able to respond to changes in  .
.
To our knowledge, this is the first study of a HFNC device with closed-loop oxygen control in adults and is the largest study to date of a closed-loop oxygen-control device during an exercise test in subjects with stable chronic lung disease. A previous crossover study compared closed-loop control using a low-flow oxygen delivery device to deliver a fixed 2-L/min flow of oxygen and a fixed 2-L/min flow of compressed air in subjects with COPD during an endurance shuttle walk test.12 Compared to the HFNC room air and HFNC fixed O2 interventions in this study, the mean ± SD proportion of time spent in the target range during the endurance shuttle walk test was lower for the fixed flow of air and oxygen: 18.3 ± 20.2% and 43.9 ± 34.3%, respectively. The 60.3 ± 26.7% of time spent in the target range using closed-loop control with low-flow oxygen was similar to HFNC closed loop in this study. The different methods of exercise testing, use of high-flow oxygen, and different subject and controller characteristics may explain these differences.
The strengths of this study include the use of HFNC with all interventions, ensuring that any differences observed were due to the delivered oxygen concentration rather than the effect of high-flow oxygen. Limitations include the potential for a learning effect between 6MWTs, although this is unlikely to affect oxygenation parameters and would be unlikely to introduce a systematic bias due to the randomized nature of the study. In addition, the modest flow of 35 L/min may have reduced the ability to achieve optimal oxygenation during exertion. The confidence intervals for outcomes relating to oxygenation were wide for all interventions, reflecting the variable responses to oxygen between subjects during the 6MWT. The target 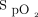 range of 92–96% is higher than would be considered necessary for the prescription of ambulatory oxygen in clinical practice, and HFNC would not typically be used in this setting; however, the aim of the study was to assess device responsiveness to changes in
range of 92–96% is higher than would be considered necessary for the prescription of ambulatory oxygen in clinical practice, and HFNC would not typically be used in this setting; however, the aim of the study was to assess device responsiveness to changes in 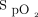 and was not intended to reflect clinical practice with regard to the use of ambulatory oxygen therapy. Given that walk tests were stopped in the event of desaturation to < 80%, we cannot determine the efficacy of closed-loop control in response to more severe desaturation. Finally, we have reported a large number of statistical tests, therefore some of the apparent differences may be due to Type I error inflation.
and was not intended to reflect clinical practice with regard to the use of ambulatory oxygen therapy. Given that walk tests were stopped in the event of desaturation to < 80%, we cannot determine the efficacy of closed-loop control in response to more severe desaturation. Finally, we have reported a large number of statistical tests, therefore some of the apparent differences may be due to Type I error inflation.
Conclusions
This study provides proof-of-concept evidence that the novel HFNC device with closed-loop control can respond to changes in  outside a target saturation range, using a model of exercise-induced desaturation and subsequent recovery. These findings warrant further investigation into their clinical utility in the acute care setting.
outside a target saturation range, using a model of exercise-induced desaturation and subsequent recovery. These findings warrant further investigation into their clinical utility in the acute care setting.
ACKNOWLEDGEMENTS
We thank the subjects for their involvement in the study, as well as Claire Richards (pulmonary rehabilitation nurse) for her assistance in identifying potential subjects.
Footnotes
- Correspondence: James CP Harper MBChB MRCP. Medical Research Institute of New Zealand, Private Bag 7902, Newtown, Wellington 6242, New Zealand. E-mail: james.harper{at}mrinz.ac.nz
This work was supported by Fisher and Paykel Healthcare and the MRINZ receives funding from the Health Research Council of New Zealand as an Independent Research Organisation grant. The authors have disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://www.rcjournal.com.
- Copyright © 2021 by Daedalus Enterprises