Abstract
BACKGROUND: The purpose of this study was to evaluate how factors (ambient temperature, shaking the inhaler before use, suspension of the inhaler in water, and the variation over the lifetime of the inhaler) affect the particle-size distributions from albuterol HFA inhalers.
METHODS: We used a laser particle-size analyzer to measure the percentage of particles in the 1- to 5-μm range (fine-particle fraction) serially 2,500 times per second to obtain a window of useful measurements with each inhaler actuation. We compared the inhaler performance results as follows: cold versus hot, full versus partial versus empty inhaler actuations, shaken versus unshaken, and inhaler characteristics after water submersion.
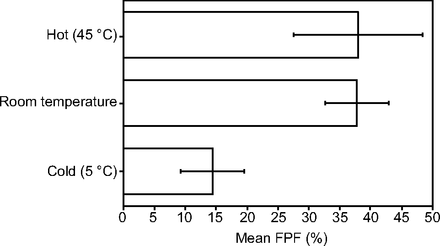
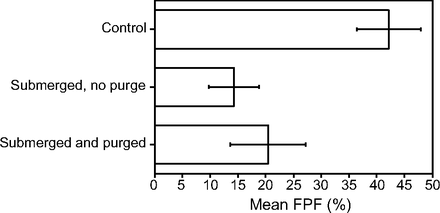
RESULTS: The effect of temperature was as follows: fine-particle fraction was 14.4% at 5°C, 37.9% at 24 - 25°C, and 38.1% at 45°C. The fine-particle fraction at the start, middle, end, and past the end of the inhaler’s rated lifetime were 37.9, 26.3, 27.9, and 22.0%, respectively. Shaking the inhaler did not improve the inhaler’s fine-particle fraction. Submerging the inhaler reduced the fine-particle fraction to 14.3% without purging and to 20.5% with purging compared with the 42.1% for the control inhaler, which was not submerged.
CONCLUSIONS: Temperature made a difference, with cold inhalers producing a lower fine-particle fraction. The early portion of the inhaler had a better fine-particle fraction than the middle and end of the inhaler’s lifespan. We could not demonstrate that shaking the inhaler had a significant effect on the fine-particle fraction. Submerging the inhaler in water significantly reduced the fine-particle fraction.
Introduction
The known ideal particle size for albuterol (also known as salbutamol) for inhaled bronchodilation and deposition in the smaller airways is between 1 and 5 μm, which makes this a critical factor in therapeutic efficacy. Albuterol pressurized metered-dose inhalers used chlorofluorocarbon propellants, which were banned internationally. Chlorofluorocarbon propellants were replaced by hydrofluoroalkane (HFA) propellants in 2008. Inhaler technique is a significant therapeutic problem,1-3 despite attempts to improve technique.4-6 Factors that potentially affect particle sizes have not been well studied in HFA inhalers because most of the previous studies were conducted on chlorofluorocarbon-containing pressurized metered-dose inhalers. Therapeutic recommendations to optimize albuterol HFA inhaler efficacy are commonly given to patients, sometimes without robust evidence that these recommendations are in fact correct. The purpose of this study was to evaluate how these factors (ambient temperature, shaking the inhaler before use, suspension of the inhaler in water, and the variation over the lifetime of the inhaler) affect the particle-size distributions from albuterol HFA inhalers (not the total emitted dose of albuterol).
Quick Look
Current Knowledge
There are numerous recommendations for inhaler use, many of which are substantiated in the older chlorofluorocarbon inhalers but many of which are not substantiated with HFA albuterol inhalers. All HFA albuterol inhalers have slightly different additive contents, which makes the HFA conversion even more complex.
What This Paper Contributes to Our Knowledge
Some of the inhaler-use recommendations were assessed on the Ventolin brand albuterol HFA inhalers by using a laser particle sizer that demonstrated that cold temperatures, older life span of the inhaler, and submerging the inhaler in water, all reduced the inhaler’s fine-particle fraction. We could not demonstrate that shaking the inhaler had a significant effect on the fine-particle fraction, but this finding was not sufficient to conclude that shaking has no benefit.
Methods
We used a sophisticated laser particle-size analyzer (Spraytec; Malvern Instruments, Worcestershire, United Kingdom)7 to measure the percentage of particles in the 1- to 5-µm range (fine-particle fraction) serially, 2,500 times per second, to obtain a window of useful measurements with each inhaler actuation (puff). Because this study had no human subjects, institutional board review was not required. We purchased fifteen 18-g albuterol HFA inhalers (Ventolin brand, GlaxoSmithKline, Brentford, Middlesex, United Kingdom) from a retail pharmacy at $78 per inhaler. We fixed the position of the inhaler (with the canister placed vertically, with the mouthpiece inferior to the canister) and adjusted the laser sampling window along the path of the visible albuterol puff until we obtained relatively consistent measurements, which were ∼10 cm downstream from the inhaler mouthpiece.
The laser particle analyzer wrote a row of data to a spreadsheet every 0.0004 s, or 2,500 Hz. Each column in that row contained the percentage of particles that were in a particular size range. We summed the percentages in the range 1–5 μm to obtain the total percentage of particles that were in the 1–5 µm range (fine-particle fraction). Before the inhaler puff and after the puff passes the sampling window, the values record a series of zeros. Even during the valid sampling period, some of the data reads were not valid, which resulted in a row of data that contained mostly zeros. To obtain the best mean value from these serial measurements and to prevent the invalid data rows from affecting the overall mean, macro code was written to automatically evaluate each line of data. If the 1–5 µm percentages summed to > 1%, this was considered a valid data row. We compared the inhaler fine-particle fraction performance results for the following performance factors: cold versus hot, full versus partial versus empty inhaler actuations, unshaken versus shaken, and inhaler characteristics after water submersion. We created protocols to study each of the performance factors.
To study the effect of temperature on inhaler performance, we cooled an inhaler to 5°C and warmed a different inhaler to 45°C, and obtained serial measurements. The control inhaler was kept at room temperature (24°C-25°C). To study the effect of inhaler performance during its lifetime, we obtained measurements at the beginning, middle, and end of its lifetime based on the puff counter on the inhaler. We continued to test the inhaler past the 0 puffs remaining point. To study the effect of shaking an inhaler before its use, we used 2 separate inhalers. One was not shaken and the other inhaler was shaken. There was a large degree of variance in the shaken inhaler, thus we proceeded to gather more data with a single new inhaler to avoid any differences between the inhalers. We measured a puff unshaken. We then shook the same inhaler for 1 min and measured a puff. We then left the inhaler on a shelf for a minimum of 10 h. We repeated the above procedure in the same fashion to alternate unshaken and shaken measurements to avoid the confounding effect of the early versus the late portion of the inhaler’s life.
To study the effect of submerging an inhaler in water, which was an older recommendation to determine whether the inhaler is full or empty, we submerged the canister portion of the inhaler in water. Because the inhaler was new, it sank to the bottom. We retrieved the inhaler, re-assembled the inhaler, then measured its performance without purging (submerged, no purge) the inhaler first. We then purged the inhaler and measured its performance again (submerged and purged). This was compared with a control inhaler that was not submerged or purged.
Results
The means ± SDs for the effect of temperature on inhaler performance, which showed that a cold inhaler performed poorly compared with a room temperature and a warm inhaler are summarized in Table 1. This same comparison is graphically shown in Figure 1. The same data for the inhaler’s performance at the beginning (full), midway, at the end (nearly empty), and beyond empty state are summarized in Table 2. The fine-particle fraction was better in the beginning compared with the middle, end, and past the end of the inhaler’s capacity (lifespan). This same comparison is graphically shown in Figure 2. The same data for the inhaler’s performance when not shaken compared with when it was shaken are summarized in Table 3. We initially used 2 separate inhalers, but the variance of the shaken inhaler was much larger than the non-shaken inhaler. At that point, we suspected that there was a difference between the inhalers. Thus, we proceeded to compare non-shaken versus shaken in a new single inhaler by alternating the measurements as described in the methods section. In this method, the mean fine-particle fraction values were roughly the same.
Fine-Particle Fractions at Hot, Room, and Cold Temperatures*
Mean fine-particle fraction (FPF) at hot, room temperature, and cold temperatures. The error bars represent the SDs.
Mean fine-particle fractions (FPF) at the beginning (full), mid life, end (empty), and past the end of the inhaler’s life. The error bars represent the SDs.
Fine-Particle Fractions at the Beginning (Full), Mid Life, End (Empty), and Past the End of the Inhaler’s Life*
Fine-Particle Fractions From a Non-shaken Inhaler Vs a Shaken Inhaler*
However, the overall numbers showed lower fine-particle fraction values than the previous inhalers, despite the data being obtained from the beginning portion of a new inhaler. Nevertheless, both comparisons were unable to show a difference between non-shaken and shaken measurements. This same comparison is graphically shown in Figure 3. The top comparison bars were not visibly similar, but the SDs were large and the P values were not significant. The lower comparison showed bars that were nearly the same. This indicated no improvement in fine-particle fraction with shaking the inhaler. The same data for the inhaler’s performance after submersion in water without purging and with purging compared with a control inhaler that was not submerged in water are summarized in Table 4. This showed poor inhaler performance after submersion, which partially recovered after purging (puffing) the inhaler. This same comparison is graphically shown in Figure 4.
Mean fine-particle fractions (FPF) at the beginning of an unshaken, then shaken inhaler. The error bars represent the SDs. A: Graphs compare 2 different inhalers not shaken versus shaken. B: Graphs compare alternating not shaken and shaken measurements from a single inhaler.
Fine-Particle Fractions for a Submerged Inhaler Without Purging and With Purging Compared With a Control Inhaler Not Submerged in Water*
Mean fine-particle fractions (FPF) in a control unsubmerged inhaler compared with an inhaler that was submerged in water (with no purging) and a submerged inhaler after purging. The error bars represent the SDs.
Discussion
This study demonstrated differences in inhaler fine-particle fraction with temperature, the fullness of the inhaler, and after submersion. Temperature made a difference, with cold inhalers producing a lower fine-particle fraction. Patients who reside in cold climates should keep the inhaler warm to optimize its performance. The early portion of the inhaler lifespan had a better fine-particle fraction than did the middle and the end. It is unclear what this means clinically. It should be noted that the laser particle-size analyzer is only able to measure particle sizes as a resulting percentage of particles in each size category. It does not indicate the composition of the particles or the milligrams of the albuterol particles, and it does not count the number of particles. Thus, it is possible that, when the inhaler is full, the higher percentage of 1–5-µm particles could be due to better inhaler performance, or it could be due to some kind of artifacts such as propellant particles. The HFA propellant is supposed to evaporate rapidly so that it does not form particles; however, close-distance test fires of the inhaler into paper towels did result in a quickly evaporating wet mark. Another study that measured the total amount of the drug ejected from the inhaler showed higher doses of albuterol ejected from the beginning of the inhaler, with declining doses as the inhaler advanced through its life cycle, which corroborated the results from our study.8
We were unable to demonstrate significant differences in the inhaler fine-particle fraction with shaking versus non-shaking. Thus, it could have been that the total contained albuterol particles were lower without shaking the inhaler, even though the percentages were the same because the laser size analyzer measures the percentage of particles and not milligrams of albuterol particles or total particles. Two studies showed that the time between shaking and firing affects the total dose emitted from the inhaler, with increasing doses emitted with increasing time delays from 0 to 60 s after shaking (ie, more drug was emitted 60 s after shaking compared with 0 s after shaking).8,9 In addition, other drug inhalers, such as corticosteroid inhalers, long-acting β-agonist inhalers, and combination inhalers, perform differently with shaking, which seems to depend on whether the drug is denser than the propellant.8,9
Submerging the inhaler in water significantly reduced its fine-particle fraction. Currently, albuterol inhaler products all come with a counter. In the past, some albuterol inhalers did not come with puff counters and, thus, submersion in water was a recommended procedure to assess how much albuterol remained in the canister. From our study, this practice should be discouraged and it seems to be largely unnecessary because all current inhalers have puff counters.
In comparing this with albuterol by wet nebulizer in which albuterol is nebulized from an aqueous solution, the albuterol particles from an HFA inhaler are not aqueous. We trapped the albuterol HFA inhaler particles on a microscopic glass slide and photographed them. We sized these particles under magnification to confirm that the laser particle analyzer was yielding similar results, which confirmed its proper functioning and the validity of this measurement method. These microcrystalline albuterol particles are not spherical as would be expected in nebulization of aqueous albuterol solution. Thus, although they might be measurable by the laser particle-size analyzer, a 3-µm particle, was not likely to be a 3-µm sphere. An elongated particle compared with a spherical particle of the same volume will have different drag force characteristics, thus, it might be possible that the optimum particle size of 1 to 5 μm might not be applicable to albuterol particles delivered via HFA inhaler. Furthermore, the method with which the particle sizer analyzes particles assumes spherical shapes. An elongated particle, depending on the moment it strikes the laser, may register as 2 different size particles because the detectors of the sizer are arranged on only 1 axis (eg, a particle entering the beam with the thin dimension on the horizontal axis will register as a small-diameter particle, whereas that same particle entering with the long dimension on the horizontal axis will register as a large-diameter particle).
Albuterol inhalers are not chemically identical. This study used the Ventolin brand of albuterol inhaler. The package insert of the ProAir inhaler (Teva Respiratory, LLC, Frazer, PA) states that it contains ethanol in addition to microcrystalline albuterol sulfate and HFA. The package insert of the Proventil inhaler (Schering Corporation, a subsidiary of Merck & Co., Whitehouse Station, NJ) states that it contains ethanol and oleic acid. The package insert of Ventolin does not mention the presence of ethanol or oleic acid. A study by Johnson et al,10 concluded that these 3 inhaler products should not be considered interchangeable because they perform differently. Thus, the results from this study apply to the Ventolin products only and might not be applicable to the other inhaler products.
To summarize the limitations discussed above: (1) differences in inhaler brands, (2) comparisons were based on the percentage of particles in the 1–5-µm range and not the total number of particles in this size range, (3) the non-spherical shape of the inhaler’s albuterol particles, and (4) the use of the Ventolin brand inhaler, which might be different from other albuterol inhaler brands. Other studies have examined additional clinical factors that affect respiratory drug deposition, such as humidity11,12 and insertion angle of the inhaler,11 thus, there are numerous other factors that affect inhaler performance and clinical response that were not tested in our study.
Conclusions
The best albuterol inhaler fine-particle fraction was obtained at room temperatures, with a full albuterol canister (new inhaler early in its lifespan). Cold temperatures, older inhalers, and submersion of the albuterol canister in water all resulted in poorer fine-particle fractions. We could not demonstrate any consistent effect on the fine-particle fraction from shaking the albuterol HFA inhaler.
Footnotes
- Correspondence: Loren G Yamamoto MD, Department of Pediatrics, University of Hawaii John A. Burns School of Medicine, 1319 Punahou Street, 7th Floor, Honolulu, HI 96826. E-mail: Loreny{at}hawaii.edu
This study was supported by a grant from the Hawaii Pediatric Association Research and Education Foundation
- Copyright © 2021 by Daedalus Enterprises