Abstract
Mechanical ventilators display detailed waveforms which contain a wealth of clinically relevant information. Although much has been written about interpretation of waveforms and patient-ventilator interactions, variability remains on the nomenclature (multiple and ambiguous terms) and waveform interpretation. There are multiple reasons for this variability (legacy terms, language, multiple definitions). In addition, there is no widely accepted systematic method to read ventilator waveforms. We propose a standardized nomenclature and taxonomy along with a method to interpret mechanical ventilator displayed waveforms.
Introduction
A wise man once said that which is simple is rarely seen, and that which is seen is seldom understood. What to say about that which is complicated?
The ventilator waveforms provide us with a continuous stream of information on both physiology and patient-ventilator interactions. This information is invaluable for patient care. As critical care providers, we need to change how we see the mechanical ventilator screen. Too often, training on ventilator waveforms is based on simple pattern recognition (eg, double triggering, missed trigger) and ends up being just an experience-based exercise rather than a systematic process.
A physician in training spends countless hours learning how to read EKGs. The electrical manifestations of the heart’s activity provide a window into the heart’s physiology and function. Knowing how to read an ECG is a key skill that all clinicians must master. In daily clinical practice, we have continuous ECG monitoring at the bedside that provides important information. Many centers have individuals monitoring continuously to recognize dangerous patterns. We print, interpret, and document ECG strips every shift in our ICU. Comparing this to our practice with ventilators, the deficit in our approach immediately becomes evident. Yes, we observe the ventilator display and document settings, but we do not collect any information on the interpretation. Of course, there are clinicians around the world who do routinely read ventilator waveforms and interpret patient-ventilator interactions, but this seems to be the exception rather than the rule.
Another challenge is the plethora of terms used to describe patient-ventilator interactions. There is no standardized vocabulary to describe what we see. Review articles, research studies, and case reports use different names and definitions for patterns observed.1,2 Some use the etiology (eg, reverse trigger), the outcome (eg, breath-stacking), patterns (eg, double trigger), or ambiguous terms (eg, flow asynchrony) that we use and understand; yet, it generates problems in education, research, and scholarship.3
There are some historical and practical reasons for our practice deficit. First, early ventilators did not provide graphical displays. Second, the nature of the display, being an instantaneous image and having no printout, makes it harder for us to document and analyze the waveforms. (Some ventilators allow screen captures to be saved as image files, but this is far from convenient.) Finally, and perhaps most important, there was never a widely accepted, formal, systematic method to read these waveforms as there is for ECGs.4 Most of us have relied on self-study, experience, and learning from mentors.
Here we propose a standardized nomenclature and taxonomy along with a method to read ventilator waveforms based on knowledge of modes of mechanical ventilation, the physiology of the respiratory system, and the analysis of interactions of patient with the ventilator. The method has 3 steps, which lead to complete analysis of the waveforms.
Step 1. Define the TAG for the Mode (What the Mode Actually Does)
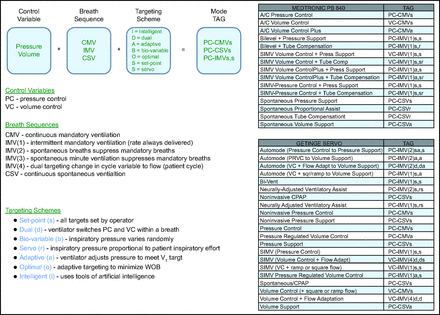
To define a mode, you must learn a small but specialized vocabulary of standardized terms and the taxonomy used to classify modes. We have written extensively on this topic.5-7 Briefly, a mode can be identified as a specific combination of 3 components, the control variable, the breath sequence, and the targeting scheme (Fig. 1). We summarize these 3 components with an abbreviation called the taxonomic attribute grouping (TAG). Clinicians can classify modes of any ventilator using the taxonomy.5 We have classified all the modes on almost every ventilator used in the United States.8 The mode taxonomy itself has been described in most current textbooks that contain chapters on mechanical ventilation.9-14 We maintain an evolving public database containing every mode on every ventilator (see related supplemental material at http://rc.rcjournal.com). Also, a subset of these modes, for ventilators at our hospital, has been placed on a pocket card that we call the TAG Mode Table (Fig. 1).
Summary of ventilator mode taxonomy and sample of a taxonomic attribute grouping (TAG) mode card. A mode can be described by 3 components: breath control variable, breath sequence, and targeting scheme. We use a TAG to summarize these features. We can classify all modes present on a ventilator using a TAG mode card, which allows rapid identification of each mode’s features. WOB = work of breathing, SIMV = synchronized intermittent mandatory ventilation.
There are several reasons why classifying a mode is an essential step, but the main one is that the commercial name of the mode does not necessarily represent the mode’s actual behavior. The classic example that highlights this is the mode named pressure regulated volume control (VC) (on Getinge Servo ventilators), which is often thought to be a VC mode.15 It is available on many other ventilators but with different proprietary mode names (eg, VC plus or volume guarantee) or can be activated under another mode name (eg, VC + auto flow on the Dräger V500 ventilator) with the name of the mode not even changing. All these modes have the same TAG, PC-CMVa, that tells us the control variable is pressure (PC), the breath sequence is continuous mandatory ventilation (CMV), and the targeting scheme is adaptive (a). We will describe all these further below, but the point is that to be able to read the ventilator waveforms we must understand what the ventilator is programmed to do. The TAG tells you that. It defines the mode’s 3 components (control variable, breath sequence, and targeting scheme) that help you analyze the waveforms to read the patient physiology (step 2) and patient-ventilator interaction (step 3).
Step 2: Determine the Load
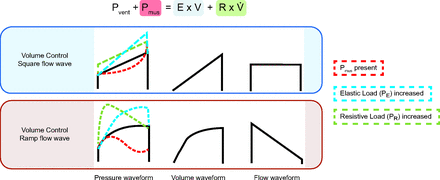
The ventilator’s pressure, volume, and flow waveforms contain important information on the physiology of the respiratory system. Without any other intervention, just by observation of the waveforms, the clinician can determine the main loads of the respiratory system (Fig. 2).
Graphic representation of the equation of motion and loads on ventilator waveforms. Notice, for all modes, at the beginning of the breath, the main load is resistive; at the end of the breath, the elastic load predominates. Pmus, = pressure generated by the patient; Pvent = pressure generated by the ventilator; E = elastance; V = volume; R = resistance; V̇ = flow; PR = resistive load; PE = elastic load.
During inspiration, the respiratory muscles or/and the ventilator generate pressure to deliver flow and volume against the counterpressure from the resistive and elastic properties of the respiratory system. The mathematical model describing this is the equation of motion for the respiratory system. There are many forms of this equation, but a simple version is expressed as
 (1)where Pvent is the inspiratory pressure generated by the ventilator (above PEEP), Pmus is the inspiratory pressure generated by the respiratory muscles, E is elastance (cm H2O/mL), V is volume (mL), R is resistance (cm H2O/L/s), and V̇ is flow (L/min), all measured relative to their end-expiratory values. The term E × V has the dimensions of pressure and is called the elastic load (PE, the force to expand the chest wall and lungs). The term R × V̇ also has the dimensions of pressure and is called the resistive load (PR, the force to cause flow through the airways, both natural and artificial). Pvent and/or Pmus has to overcome the elastic load and resistive load to achieve movement of air.
(1)where Pvent is the inspiratory pressure generated by the ventilator (above PEEP), Pmus is the inspiratory pressure generated by the respiratory muscles, E is elastance (cm H2O/mL), V is volume (mL), R is resistance (cm H2O/L/s), and V̇ is flow (L/min), all measured relative to their end-expiratory values. The term E × V has the dimensions of pressure and is called the elastic load (PE, the force to expand the chest wall and lungs). The term R × V̇ also has the dimensions of pressure and is called the resistive load (PR, the force to cause flow through the airways, both natural and artificial). Pvent and/or Pmus has to overcome the elastic load and resistive load to achieve movement of air.
A note to the reader: Elastance is the inverse of compliance. When we say high elastance, it means low compliance and vice versa. They are used interchangeably throughout the text. For example, we use elastance when referring to the equation of motion and compliance when discussing the time constant as it simplifies the mathematics. For interpreting waveforms, we use compliance as this is what most clinicians use at the bedside.
Having defined the concept of load, we will now describe the process for identifying the dominant load (resistive or elastic), or if there is evidence of patient effort (Pmus), which makes determination of resistance and elastance difficult. To be able to read the load or identify the presence of Pmus, we start by identifying the breath control variable (it is defined in the TAG). The breath control variable is the output the ventilator controls, either pressure or volume.
In VC modes, by definition, the ventilator controls the inspiratory flow waveform (ie, the operator sets the peak flow and tidal volume), so the resulting pressure is the manifestation of the respiratory system resistance and elastance. Thus, in VC you see the load and patient interactions in the pressure waveform. Ventilators are excellent at controlling flow, so the tidal volume and flow waveform will not change in the presence of Pmus for VC with set-point targeting (see below).
In contrast, for pressure control (PC) modes, by definition, the inspiratory pressure waveform is controlled by the ventilator (either the inspiratory pressure target is preset before inspiration starts or the ventilator adjusts inspiratory pressure to be proportional to the patient’s inspiratory effort during inspiration), so the resulting flow and volume are the manifestation of resistance and elastance of the respiratory system. Thus, in PC you see the load and patient interactions in the flow and volume waveforms. Ventilators are mediocre at controlling pressure (compared to controlling flow), so some changes may also be evident in the pressure waveform, but the flow waveform should be the focus.
Note to the reader: When assessing load or patient-ventilator interactions, attention should be focused to the waveform opposite the control variable.
The Anatomy of Ventilator Waveforms
To understand the physiology (determine the load), it is useful to know the determinants of the pressure, volume, and flow waveforms for VC and PC during passive mandatory breaths, as in Figure 2. By passive, we mean that Pmus = 0; and by mandatory, we mean that inspiration is either started or stopped (triggered or cycled) by the ventilator. The waveforms that contain the information on the elastic and resistive loads have the dimensions of pressure. Actually, these are graphical representations of the equation of motion. At any moment in time (horizontal axis of the graphs), the height of the pressure waveform is simply the height of the volume waveform plus the height of the flow waveform (appropriately scaled by E for volume and R for flow) because Pvent = PE + PR. Every ventilator that displays pressure, volume, and flow waveforms is plotting the equation of motion (Fig. 2). This knowledge is useful to understand how the resistive and elastic loads manifest in the waveform according to the mode. Here we describe the most common patterns.
Volume Control - Square Waveform
Figure 2 depicts the pressure, flow, and volume waveforms for a VC passive inspiration with a square flow waveform (ie, constant flow). If we consider the resistance and elastance to be constant through the inspiration because the flow and volume are controlled, the resultant pressure waveform will be a manifestation of the patient’s elastance and resistance.
Effects of Resistance.
In VC square waveform (VCsq), the ventilator delivers a constant inspiratory flow. It rises immediately and stops at the end of the inspiratory time. This constant flow from the ventilator first encounters the resistance of the natural and artificial airways. Because inspiration starts with an immediate rise in flow from zero to the set value, and essentially no volume delivered, the airway pressure rises immediately as a manifestation of the resistive load (R × V̇). Once volume starts to be delivered to the alveoli, the airway pressure becomes a manifestation of both elastic and resistive loads. At the end of the inspiration, in the absence of an inspiratory hold, the airway pressure is the result of both the resistive and elastic loads (ie, the end-inspiratory pressure). Figure 3 demonstrates that the resistive load is constant throughout the inspiration (because flow and resistance are constant). If the flow stops (manual or preset inspiratory hold), the resistive load is zero (R × 0 = 0) and only the elastic load remains; this is the classic way taught to evaluate the loads. You now can identify this with or without an inspiratory hold: The higher the resistive load, the higher the initial step up in pressure; and if there is an end-inspiratory pause, the greater the fall from the peak to the plateau pressure.
Effect of changes in Pmus, resistive and elastic loads in volume control. The flow and volume waveforms do not change as these are controlled by the ventilator. Changes in resistive load affect the initial step in Pvent without a change in slope. Changes in elastic load change the slope of pressure rise. Patient effort (Pmus) adds or subtracts to the airway pressure; in this case, patient inspiratory effort subtracts to the airway pressure.
Because the determinants of resistive load are flow and airway resistance, as either flow or resistance increases the resistive load will increase. Consequently, the inspiratory resistive load can also be high by setting high inspiratory flows (often done to decrease inspiratory time). To query if the issue is resistance or high flow, it is useful to evaluate the expiratory flow waveform (see section below) as generally high resistance will manifest both during inspiration and expiration.
Effects of Compliance.
In VCsq, the volume is delivered at a constant rate (flow) to the lung, and the airway pressure rises linearly. The slope of the pressure rise is proportional to elastance. The higher the elastance (the stiffer the lungs) the faster the pressure rises. The equation of motion assumes that elastance is constant. That is usually the case; however, in patients with very sick lungs (eg, ARDS), the elastance may change during inspiration. If there is recruitment during inspiration, the elastance would decrease and so would the slope. On the contrary, if during inspiration there is overdistention, the elastance would increase, leading to an increase in slope. The stress index16 is a mathematical equation used to detect nonconstant elastance by changes in the slope of the pressure waveform during VCsq. This is useful only if the patient is passive because any Pmus can distort the pressure waveform in VC.
Effect of Pmus.
In VCsq, the Pmus will add to (inspiratory effort) or subtract from (expiratory effort) the inspiratory force of Pvent. The pressure waveform will be deformed accordingly. During inspiration, if the patient makes an inspiratory effort, then the pressure waveform will show a concave upward deformation. Patient effort can be short, long, of different intensities, and may occur early or late in the breath. It is not possible to reliably discern the elastic or resistive loads while the Pmus occurs (see work shifting below) (Fig. 3).
Volume Control Descending Ramp Waveform
In VC descending ramp waveform (VCdsc), flow starts at the preset peak value and then decreases in a linear fashion through the inspiratory time. The slope of the ramp depends on the inspiratory time, the set peak flow, and set end-expiratory flow. It is important to remember that not all descending ramp waveforms are the same. On some ventilators, end-expiratory flow is set to a default value of zero, whereas on others it can be set to a percentage of the peak flow (it is unclear why a manufacturer would give this as an option). In some ventilators, the time to reach peak flow may also be modified (called adjustable rise time, with a similarly unclear rationale for this option). For this discussion, we will use examples where inspiration begins at peak flow and ends at zero flow.
Effect of Resistance.
Similar to the square flow, the flow from the ventilator first encounters the resistance of the natural and artificial airways. The airway pressure rises immediately as a manifestation of the resistive load. Figure 3 depicts the classic changes in pressure due to increased resistive loads. One may see waveforms suggestive of high resistance when the flow is set high or when the resistance is high. To determine if the issue is resistance or high flow, examine the expiratory flow waveform to evaluate for airway resistance.
Effect of Compliance.
In VCdsc, after flow begins, the effects of compliance will be hidden by the resistive component just as with VCsq. The difference is that at end inspiration (assuming flow goes to zero) the end-inspiratory pressure is all PE. In this case, the end-inspiratory pressure is essentially the same as plateau pressure after an inspiratory hold. In Figure 3, you can observe that if the elastic load is high (poor compliance) then the end-inspiratory pressure is higher. With this bedside observation, you can assess if the elastic or resistive pressures are elevated without imposing any maneuvers. If the flow is not set to end at zero, then these assumptions do not apply, and the only way to assess the elastic load is an end-inspiratory hold to measure plateau pressure.
Effect of Pmus.
The effects of Pmus on the pressure waveform during VCdsc are the same as for VCsq. It is not possible to discern the elastic or resistive loads while the Pmus occurs.
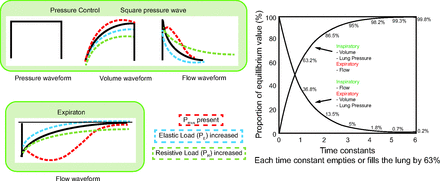
Pressure Control Waveform
In PC with a square waveform, the ventilator increases the pressure from PEEP to the set inspiratory pressure. This step increase in pressure is immediate (unless the pressure rise time setting is increased) and lasts for the set inspiratory time. At the end of inspiration, the pressure decreases immediately to the set PEEP. Figure 2 depicts the pressure, flow, and volume waveforms for a PC passive breath.
In PC, because pressure is the control variable, we look at the flow and volume waveforms to see the patient’s respiratory system resistance, compliance, and, if present, Pmus effects. In passive conditions, after a step change in pressure (up or down), the flow and volume rise or fall exponentially. The determinants of the rate of exponential change are the respiratory system resistance and compliance. The time constant (τ) is the parameter that explains this (it is derived from the solution of the equation of motion for volume and flow after a step change in Pvent). Understanding the time constant helps us deduce which load (resistive or elastic) is dominating. The formula is simple,
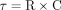 (2)where τ is the time constant (in seconds), R is resistance (in cm H2O/L/s), and C is compliance (in L/cm H2O). One time constant is the period of time where there is a 63% change in flow or volume (and hence alveolar pressure) in response to a step change in pressure at the airway opening. Suppose the pressure changes from 0 to 10 cm H2O on inspiration (or 10 to 0 cm H2O on expiration). At the end of a period of time equal to one time constant (1 × τ), the volume and flow will have changed by 63%. Hence, alveolar pressure will have changed by 6.3 cm H2O, leaving 3.7 cm H2O until equilibration). At the end of another time constant (2τ), another 63% (or 0.63 x 3.7 = 2.3 cm H2O) change will occur and so on (Fig. 4). This change continues, in theory, until infinity. For practical purposes, after a period equal to 5τ, inspiration or expiration is considered complete (< 1% of volume left). Setting inspiratory or expiratory times to at least 3τ is usually acceptable (Table 1).17
(2)where τ is the time constant (in seconds), R is resistance (in cm H2O/L/s), and C is compliance (in L/cm H2O). One time constant is the period of time where there is a 63% change in flow or volume (and hence alveolar pressure) in response to a step change in pressure at the airway opening. Suppose the pressure changes from 0 to 10 cm H2O on inspiration (or 10 to 0 cm H2O on expiration). At the end of a period of time equal to one time constant (1 × τ), the volume and flow will have changed by 63%. Hence, alveolar pressure will have changed by 6.3 cm H2O, leaving 3.7 cm H2O until equilibration). At the end of another time constant (2τ), another 63% (or 0.63 x 3.7 = 2.3 cm H2O) change will occur and so on (Fig. 4). This change continues, in theory, until infinity. For practical purposes, after a period equal to 5τ, inspiration or expiration is considered complete (< 1% of volume left). Setting inspiratory or expiratory times to at least 3τ is usually acceptable (Table 1).17
Effect of changes in Pmus, resistive and elastic loads in pressure control. The pressure waveform may demonstrate changes; however, it is being controlled by the ventilator. The time constant (RxC) describes a change of 63% per time constant in flow, volume, and alveolar pressure. The decay of the waveforms is commonly termed exponential decay and is a manifestation of a passive patient. The presence of Pmus will deform the waveforms and will not allow to determine the respiratory loads.
Example of Time Constant according to Disease
Key Concepts: The flow waveform contains mostly the same information as the volume waveform (including tidal volume, which is the area under the flow-time curve). The time constant is important when examining passive flow waveforms during expiration for VC and for inspiration and expiration with PC. The time constant is the result of a multiplication, so if the compliance or resistance is high, then the τ will be long; and if the compliance or resistance is low, then the time constant will be short. In practice, a short time constant is generally due to low compliance (when has low resistance been an issue?). A long time constant means either the resistance or compliance is high, and most of the time, clinically, the issue is resistance. In general terms, the time constant in a passive patient, intubated, using a heated humidifier with a normal respiratory system is 0.5 s, and thus the time to reach zero flow is approximately 2.5 s.17 The time constant varies according to the clinical condition (See Table 1). In general, the goal is to recognize extremes: very long or very short τ (Fig. 4).
The reader is advised that there are a few instances when PC is not delivered with a square waveform. In PC using servo targeting (eg, neurally adjusted ventilatory assist [NAVA] and proportional assist ventilation [PAV]) modes, inspiratory pressure is controlled to be proportional to inspiratory effort based on the signal related to Pmus (ie, flow or diaphragm electrical activity). Another reason is that most ventilators allow the user to set the pressure rise time. If rise time is greater than zero, then the pressure rises in a curvilinear fashion to the target, and thus inspiratory volume and flow are not simple exponential equations determined by a single time constant. Nevertheless, the passive expiratory volume and flow waveforms are unaffected.
Effect of Resistance.
At the beginning of the inspiration, the step change in airway pressure, ΔP, generates the peak inspiratory flow (ie, peak flow = ΔP/R). As volume is delivered, alveolar pressure rises. Hence, the pressure driving flow (airway pressure minus alveolar pressure) continually decreases with the resultant exponential decay in flow (determined by the time constant). The resistive load is the highest at the beginning of the inspiration and lowest at end of inspiration.
If the resistance is elevated, then the time constant is longer. You can observe that peak flow is lower and the flow waveform will take longer to reach zero flow. The same is true during expiration. Indeed, if the set expiratory time is < 3τ, flow will still be negative when the next inspiration starts, indicating gas trapping and auto-PEEP.
Effect of Compliance.
In a passive patient with no auto-PEEP, just before inspiration (when flow is zero), the alveolar pressure is equal to the airway pressure or PEEP. Immediately after the start of inspiration, the flow is increased to its maximum, and the volume starts to be delivered to the alveoli. Alveolar volume and pressure rise exponentially. Thus, the elastic load is zero at the beginning of inspiration and maximal at the end of the inspiration.
When the compliance is decreased, the time constant is decreased. Thus, the time to reach zero flow is short. A ventilated patient with normal lungs has a resistance of about 10 cm H2O (due the endotracheal tube) and a compliance of about 0.05 L/cm H2O, resulting in a time constant of about 0.5 s.17 Hence, an easy to remember rule of the thumb is that if the flow returns to zero before 1.5 s your compliance is very likely to be decreased.
Another practical tidbit: In a passive patient, if flow is zero at the end of inspiration, the end-inspiratory pressure will be equal to the plateau pressure (the static pressure that results from an end-inspiratory hold). Common misconceptions about PC are that the set target pressure is equivalent to the plateau pressure (true only if end-inspiratory flow is zero) and that a plateau pressure using an inspiratory hold can’t be obtained.
Effect of Pmus.
The presence of Pmus will theoretically deform only the flow and volume waveforms (but in practice, as mentioned above, the pressure waveform may also be distorted). The magnitude and duration of Pmus will distort the waveforms accordingly. With this distortion, you may not be able to discern the predominant load (resistive or elastic). However, if the effort is brief, and only at the start of inspiration or expiration, the distortion of the flow waveform may be insignificant enough as not to obscure the load assessment. Flow moving away from the baseline (ie, increasing flow) during inspiration indicates inspiratory effort (positive Pmus) in PC ventilation (Fig. 4). If there is evidence of Pmus during inspiration in the inspiratory flow waveform, then the expiratory waveform is your alternative to assess the physiology (as long as it is passive).
Expiration
The expiratory phase is normally passive due to the recoil of the lungs and chest wall. The expiratory phase in mechanical ventilation (ie, the period from the start of negative flow to the start of positive flow) is always pressure controlled. That is, during expiration the ventilator controls the pressure (ie, you set the PEEP). Therefore, we look at the flow (and volume) waveforms to see the physiology and patient-ventilator interactions. The driving pressure for passive expiratory flow = ΔP = Pplat, PEEP, for both PC and VC. (Note that ΔP ≠ Pplat, total PEEP because auto-PEEP only exists at end expiration, although auto-PEEP will affect Pplat.) Hence, the same concepts of the time constant apply: a long time constant means high resistance, and a short one means low compliance (Fig. 4).
The respiratory system has 2 time constants, inspiratory and expiratory, as compliance, and especially resistance, is usually not the same during inspiration and expiration. However, in the majority of cases, both move in the same direction. That is, if you see features of decreased compliance or increased resistance in the inspiratory waveform, you are likely to see them in the expiratory waveform too. We use the expiratory flow waveform to confirm the inspiratory waveform findings or when there is Pmus in inspiration. Rarely will you see them to be discordant, and if that is the case, look for something causing variable obstruction, circuit issues, or ventilator settings. For example, a normal resistive load during inspiration and high resistive load during expiration may point to an issue with the expiratory valve or expiratory filter.
The output from step 2 is to define what the dominant load (resistive or elastic) is or there is Pmus. This allows to move to the next step understanding the ventilator mode and the patient physiology.
Step 3: Diagnose the Patient-Ventilator Interaction
The last clinically important observation that can be obtained from the waveforms is how the patient and ventilator interact. The interactions occur breath by breath, and as such, they will change with patient condition, level of awareness, position, etc. Many interactions will be temporary, harmless, and may be irrelevant clinically; others may be harmful, especially if frequent (eg, failed trigger). Although many published reviews have described different types of interactions, there has been little consistency among them and no attempt at a standardized vocabulary or taxonomy. Therefore, we propose the following classification system that is based on word etymology, respiratory physiology, and a description of the interactions. The goal is that by using a standard and etymologically correct vocabulary we will provide persistence of meaning to the terms that can translate across languages; by using descriptive terms, based on signal analysis, rather than etiology, we intend to avoid ambiguity when reporting. By eliminating the cause from the name, we allow the nomenclature to remain valid as we discover other causes. Table 3 summarizes the standardized taxonomy and multiple names used in the literature. The supplemental appendix provides a glossary with the terms, their roots, and rationale.
We have split the assessment of patient-ventilator interactions into 2 general types: (1) synchrony, which refers to timing of Pvent in reference to Pmus; and (2) work of breathing, which refers to the distribution of work from the ventilator (due to Pvent) in relation to that of the patient (due to Pmus).
Synchrony means a simultaneous action, development, or occurrence. In mechanical ventilation, it refers to the timing of Pvent and Pmus signals in relation to each other. The patient activity is the reference event. The accepted standard for patient action (Pmus) is represented either by an esophageal pressure waveform (Pes) or electrical signal from diaphragm (EAdi eg, as measured during NAVA). In the absence of these monitoring systems, we use the ventilator waveforms to infer the presence of Pmus. See Table 2 for the definitions of synchrony, asynchrony, and asynchrony.
Definitions of Patient-Ventilator Interactions
Work of breathing refers to both the patient and ventilator work and their relation. This work relationship has been interpreted by some as need for more flow being delivered by the ventilator. In extreme cases, this is described as flow starvation. However, it is more accurate to use the term work shifting. Work is performed when inspiratory volume is delivered under pressure (ie, the higher the pressure and the larger the volume, the higher the work) (see work shifting section).
Note: If the patient is paralyzed/deeply sedated and there is no Pmus, then by definition there can’t be an issue with synchrony: it is a matter of operator settings choice.
Here we outline an organized method for systematic observations to assess patient-ventilator interactions. It follows the 4 phases of a breath: trigger, inspiration, cycle, expiration (Table 3).
Taxonomy of Patient-Ventilator Interactions
Trigger
The trigger event (start of mechanical inflation) is assessed in terms of synchrony (timing) with the start of the patient’s inspiratory effort. It can occur early (before the patient signal), on time (synchrony), or late (a clinically important delay). There are 2 other conditions that are not related to timing but rather to ventilator function: false trigger, where a non-Pmus signal triggers an inspiration; and a failed trigger, where Pmus fails to trigger inspiration.
Normal (Synchrony)
The ventilator responds to a patient trigger effort with a delay that is clinically unimportant. Most ventilators today have a very short trigger delay (ie, period from the start of trigger effort to the start of flow delivery, sometimes defined as the delay from pressure drop below PEEP to return to PEEP).18 This is the most common trigger interaction seen (Figure 5). Normal trigger is defined by absence of any other abnormalities described below, as it is impractical to measure trigger delay at the bedside.
Classification of trigger patient-ventilator interactions. Mode: PC-CMVs. Pvent: airway pressure waveform displayed by ventilator. Pmus: patient-generated pressure waveform, simulated, overlay to demonstrate effect across waveforms. Vertical white dotted lines are for reference of the start of the Pmus. Normal trigger: minimal drop in pressure with immediate pressurization. Late trigger: note flow crossing baseline and a prolonged drop in pressure below baseline. Early trigger: machine-triggered breath followed by evidence of patient effort (rise in flow above baseline). False trigger: patient-triggered breaths; however, no evidence of Pmus, in this case triggered by circuit leak. Failed trigger: Pmus does not trigger a mechanical breath. Pmus is manifested as flow waveform moving toward baseline and a concomitant drop in airway pressure. PC = pressure control, CMV = continuous mandatory ventilation, s = set point targeting.
Late Trigger
The ventilator responds to a patient trigger effort with a delay that may have important clinically implications. In current modern ventilators, when trigger sensitivity is set appropriately, the trigger lag is < 100 ms.18 This mainly occurs with inappropriate sensitivity settings (making it too hard to trigger). In general, flow triggering results in less effort (trigger work) for the patient than pressure triggering.18 Late trigger is recognized when there is evidence of Pmus (either a drop in baseline pressure or a rise in flow above baseline) well before the start of inspiratory flow from the ventilator. Most current ventilators will indicate patient triggering by pressure or flow by a color change in the trigger phase of the corresponding waveform.
Another trigger observation, which is not necessarily related to the trigger being late, but manifests similarly, is when the pressurization rate of the ventilator after trigger is not fast enough. This situation occurs when the ventilator pressurization rate can’t match the patient effort or, more commonly, an operator sets inappropriately long inspiratory rise times (Figure 5). Both these situations, late trigger and delayed pressurization, will cause a prolonged trigger phase and increased work of breathing early in inspiration.18,19 For simplicity, we lump them both under the same classification.
Early Trigger
A machine-triggered inspiration precedes the patient trigger effort. The key finding is the start of inspiratory flow followed by evidence of Pmus, which may or may not trigger another breath (Figure 5). The patient effort may occur any time during inspiration or early during expiration. This was initially described20 as reverse trigger, meaning that inspiratory flow from the ventilator somehow stimulated an inspiratory effort from the patient, the reverse of the normal situation. It seems there are different causes and variants, and a fair amount of work on its physiology has been described, but the phenomenon is still being clarified.21-23 We use the term early trigger, as it describes the event in terms of the signals, not the physiology, and thus it avoids changes in the taxonomy in the future. It is likely that many causes for early trigger will be found.
False Trigger
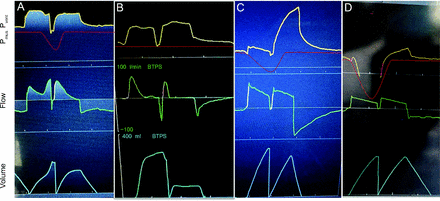
Inspiration is triggered by a non-Pmus signal (Figure 5). These (pressure or flow) signals are generally caused by secretions or fluid in the circuit/endotracheal tube/airway, cardiac oscillations, etc. False trigger can also be caused by a leak in the patient circuit. This can be challenging to recognize. Some clues are the absence of evidence of Pmus during inspiration, presence of apparently patient-triggered breaths in heavily sedated or paralyzed patients, or high-frequency oscillations in the flow waveform. Continuous capnography can provide a clue, as it will demonstrate oscillations in the expiratory waveform tracing (Figure 6, panel B). There are 2 techniques to help diagnose this. First is to assess Pmus in the patient. Observe the neck muscles for evidence of effort, look at the abdomen, and place your hand on the patient. The second is to do an end-expiratory pause and observe for patient effort and for negative deflections in the airway pressure, a manifestation of Pmus.
Capnography as an aid in recognizing asynchrony. In panel A, the capnography demonstrates a deformation during exhalation consistent with a failed trigger. The waveform moves toward baseline, demonstrating an inspiratory effort. The vertical dashed line demonstrates the timing matching the deformations in pressure, flow, and CO2 waveforms. In panel B, images from a patient who had false trigger due to cardiac oscillations transmitting to the airway. Notice capnogram demonstrates oscillations with progressive decrease of the CO2 level; this corresponds to oscillations in the flow waveform (gain increased to demonstrate the oscillations). The vertical dashed lines demonstrate the correlation electrocardiogram (ECG), capnogram and flow waveforms.
Failed Trigger
The patient effort fails to trigger inspiration. This is recognized in the expiratory flow waveform as patient-generated deflections toward the baseline that do not reach zero, a requirement for either flow or pressure triggering. It can also be recognized in waveform capnography as a downward deflection in the phase 3 expiratory plateau, often referred to as a “curare cleft” (Figure 6A). For flow triggering, the inspiratory effort must generate a positive flow higher than the sensitivity threshold (eg, 2 L/min). For pressure triggering, there must be enough positive flow to withdraw enough volume from the patient circuit to drop airway pressure below the trigger threshold (eg, 3 cm H2O). Failed trigger efforts may occur any time during the expiratory phase. Although there are many causes, the most concerning is auto-PEEP or air trapping, so we assess for other features of a high resistive load. (Note that ΔPmus during the trigger effort must exceed auto-PEEP for flow to cross zero, and this is why muscular weakness or high auto-PEEP leads to failed trigger efforts.) Other causes are over assistance (such as too large of volume delivered leading to auto-PEEP), excess sedation, and neuromuscular weakness. Naturally, a trigger threshold set too high (ie, sensitivity too low) can cause a failed trigger effort even though the effort is normal. In the waveform, high trigger threshold manifests differently from auto-PEEP, as the flow will cross zero but it will not reach the trigger threshold (Figure 5).
Inspiration
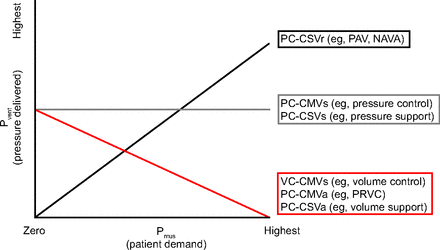
During the inspiratory phase (ie, the period from the start of positive flow to the start of negative flow), the patient-ventilation interaction is characterized by the relationship of work performed by the ventilator and the patient (Figure 7).
Relation between patient effort and ventilator-delivered pressure according to mode of mechanical ventilation. Pvent = pressure delivered by the ventilator. Pmus = pressure generated by patient respiratory effort, PAV = proportional assist ventilation, NAVA = neurally adjusted ventilatory assist, PRVC = pressure-regulated volume control. Representative sample of modes and mode names.
Work Shifting
In passive ventilation (Pmus = 0), the ventilator does all the work. In the simplest case, PC, where Pvent is held constant, work is simply the product of Pvent and VT. On the other hand, when ventilatory assistance is zero (eg, CPAP), then all the work is done by the patient (ie, Pmus generates the VT). When Pvent and Pmus are active together, some portion of the total work is done by the ventilator and some by the patient. We call this situation work shifting because some portion of the total work has shifted from the ventilator (passive case) to the patient (active case). A work shifting index could be used to quantify and characterize the relation.3 Work shifting can occur in any phase of inspiration, as it will depend when the Pmus is active, the ventilator settings (mode, inspiratory time, trigger sensitivity, cycle threshold), and patient-ventilator interaction.
The pattern of work shifting is affected by the mode and targeting scheme (Figure 7).24 In modes using VC or that use adaptive targeting schemes, the relationship is inverse (ie, as the patient does more work, the ventilator does less work, and total work remains constant). In PC modes that use set-point targeting, the relationship is such that the work output of the ventilator per liter of tidal volume stays the same as the patient work increases, although the total work increases due to the larger tidal volume. For modes that use a servo-targeting scheme, work output of the ventilator increases as the work output of the patient increases. Be aware that when work shifting becomes extreme (ie, high ventilatory drive due to hypoxemia or metabolic acidosis), this can result in either diaphragm or lung injury (ie, tidal volume overdose), and no mode or mode setting will ameliorate it. Sedation and paralysis may be required.
Volume Control With Set-Point Targeting.
During VC with set-point targeting, the operator sets the tidal volume, the peak flow, and sometimes the flow waveform. If the patient is generating inspiratory effort, the magnitude of the flow delivered by the ventilator in relation to the patient’s flow demand will affect the pressure waveform (Figure 8). The inspiratory pressure waveform will move toward the baseline as Pmus increases. Remember, in the equation of motion, Pmus and Pvent are on the same side of the equation; as the patient increases Pmus, the Pvent will have to decrease to maintain the equality with the other side of the equation, representing the pressure to deliver the tidal volume (elastic load, PE) and the pressure to deliver the flow (resistive load, PR) as shown in Figure 8.
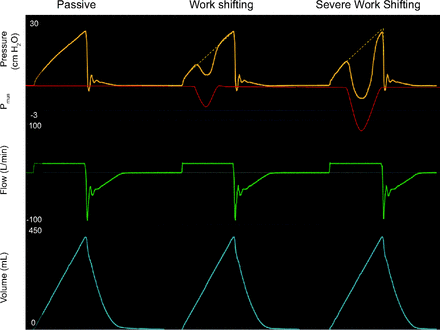
Work shifting in volume control (VC) square flow waveform. Mode: VC-CMVs. Pmus: graphic representation of patient-generated pressure. Passive breath: no Pmus. Work shifting: The pressure waveform is deformed toward baseline due to the presence of Pmus. It does not cross the baseline. Interrupted line demonstrates where the pressure waveform would be if Pmus was passive. The ventilator is still doing some amount of work on patient. It may be clinically appropriate. Severe work shifting: The pressure waveform is deformed due to the presence of Pmus; the pressure crosses the baseline (PEEP). Under this circumstance, the patient is doing work against the ventilator. This is never clinically appropriate; it needs immediate clinician attention. Note: red Pmus line was overlaid by hand onto a ventilator screen image, and Pmus is shown inverted for clarity. CMV = continuous mandatory ventilation.
The total work of inspiration stays constant because volume and flow are unaffected by Pmus (ie, the patient can maximize effort but won’t get more volume), and the total pressure (Pvent + Pmus) stays constant; hence, work, a function of pressure and volume, is unchanged. Pvent (and ventilator work) decreases in exact proportion to the increase in Pmus (and patient work). Work shifting occurs whenever the Pvent decreases in presence of Pmus. However, as long as the pressure remains above baseline (ie, PEEP), the ventilator is still performing work on the patient, and the flow delivered is still above the flow requested by the patient. In extreme cases, when the patient generates high levels of Pmus, the Pvent decreases below baseline (severe work shifting). In this case, the patient is actually doing work on the ventilator system. This extreme, commonly called flow starvation, is most often seen during VC ventilation with set-point targeting. How much work shifting is appropriate depends on the patient evaluation and clinician judgment and an area we need further research. Severe work shifting (also known as flow starvation) is never appropriate.
Pressure Control With Set-Point Targeting.
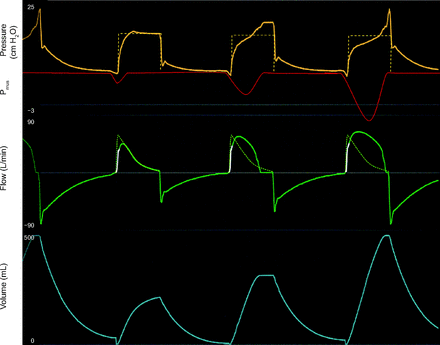
During PC with set-point targeting, the operator sets the inspiratory pressure and either the inspiratory time (CMV and intermittent mandatory ventilation [IMV]) or the flow cycle threshold (continuous spontaneous ventilation [CSV]). On some ventilators, the operator can also set the rise time, which determines the time required to reach the target pressure and affects both peak flow and tidal volume. Ideally, the ventilator should be able to achieve the target inspiratory pressure regardless of any Pmus. In practice, ventilators vary in how well they can achieve this. The presence of Pmus is identified by deformations in the flow waveform (Figure 4 and 9). Inspiratory Pmus will increase volume and flow. Because the total driving pressure (Pvent + Pmus), volume, and flow all increase, the total work increases. Furthermore, the proportion of the total work the patient does increases because Pmus increases relative to Pvent. In the presence of effort, the clinician can adjust the inspiratory pressure target up or down according to the level of ventilatory support desired. If set too low, the patient is assuming the majority of the work; and if the Pmus is large enough, airway pressure may even drop below baseline. Monitoring the patient’s ventilatory effort is helpful in this case to adjust the inspiratory support.
Work shifting in pressure control. Mode: PC-CMVs. Pmus: graphic representation of patient generated pressure. Work shifting: The pressure waveform is deformed toward baseline due to the presence of Pmus. It does not cross the baseline. Interrupted line demonstrates where the pressure waveform would be if Pmus was passive. Ventilators are unable to perfectly control the Pvent, thus the deformations. Green interrupted line demonstrates the passive flow waveform. The presence of Pmus will modify the flow waveform. In inspiration, flow will move away from baseline. Works shifting may be clinically appropriate. Notice the increase in volume as a manifestation of the added Pvent to Pmus. Note: red Pmus line was overlaid by hand onto a ventilator screen image, and Pmus is shown inverted for clarity. PC = pressure control. CMV = continuous mandatory ventilation.
Pressure Control With Adaptive Targeting.
In PC with adaptive targeting (eg, PC-CMVa, PC-CSVa, PC-IMVa,a), the operator sets a target tidal volume, and the ventilator software automatically adjusts the inspiratory pressure between breaths to achieve the target. As Pmus increases, the Pvent will decrease (Figure 10) in an attempt to maintain the VT at target.15 The relationship of work is similar to VC with set-point targeting; the difference is that in PC with adaptive targeting the total work is not constant because the tidal volume can be larger than the set target value on any given breath. The Pvent can only decrease so far (ie, PEEP or a little above depending on the design of the ventilator).15 In patients with high enough Pmus, the patient may be breathing at PEEP level with little assistance from the ventilator and larger VT than target. Some degree of work shifting may be clinically acceptable. In severe cases, this is manifested by very low Pvent (at baseline) and consistently larger tidal volumes, all generated by the patient. In some cases, the Pvent will be below baseline. Severe work shifting in adaptive targeting should be addressed promptly by the clinical team.
Work shifting in adaptive targeting schemes. Mode: PC-CMVa. Interrupted blue line demonstrates the target tidal volume. In left panel, volume is below target; the ventilator increases inspiratory pressure gradually to reach target tidal volume. In right panel, the patient effort (Pmus) leads to tidal volume above target; the ventilator gradually decreases inspiratory pressure in an aim to decrease delivered tidal volume. However, the patient effort generates larger tidal volume. The spike in pressure at the end of the breath is a manifestation of late cycle. PC = pressure control. CMV = continuous mandatory ventilation.
Pressure Control With Servo Targeting.
In PC with servo targeting (eg, NAVA and PAV), the ventilator automatically adjusts inspiratory pressure in proportion to the Pmus (Figure 7). With this targeting scheme, the ventilator keeps the relation between ventilator work and patient work constant and controllable. Work shifting is minimized and can be adjusted by setting the level of proportionality (PAV level or NAVA level). Recall that tidal volume is dependent on both the level of assistance (Pvent) and Pmus.
Cycle
Cycle (start of expiration) is assessed in terms of synchrony (timing) with the end of the patient’s inspiratory effort (ie, patient signal or Pmus). It can occur early (before the patient signal), on time (synchrony), or late (a clinically important delay) (Figure 11). Similar to triggering, there are 2 other conditions that are not related to timing but rather to ventilator function: false cycle, where a nonpatient signal cycles inspiration (eg, pressure alarm); and a failed cycle, where patient cycle signal fails to cycle the inspiration (eg, runaway phenomena). However, these manifest as early or late cycle; thus, we classified them as a cause rather than a separate patient-ventilator discordance.
Classification of cycle patient-ventilator interactions. Mode: PC-CMVs. Pvent: airway pressure waveform displayed by ventilator. Pmus: patient-generated pressure waveform, simulated, overlay to demonstrate effect across waveforms. Normal cycle: patient-triggered; the effort was small; the flow decays passively to zero flow with no evidence of inspiratory or expiratory effort. Late cycle: Green dotted line demonstrates end of patient breath; flow reaches baseline, and there is an increase of airway pressure due to relaxation of inspiratory muscles against a close valve (zero flow). Red dotted line demonstrates point where ventilator cycles. Early cycle: the machine cycles breath (red dotted line), expiratory flow with evidence of inspiratory patient effort (flow moves toward baseline). Green dotted overlay to demonstrate passive flow waveform as a reference. Note: red Pmus line was overlaid by hand onto a ventilator screen image, and Pmus is shown inverted for clarity. PC = pressure control, CMV = continuous mandatory ventilation.
Normal (Synchrony)
Inspiration ends within a clinically acceptable time near the Pmus peak (ie, Pmus increases as the diaphragm contracts and decreases as the diaphragm relaxes). In VC, we observe the inspiratory pressure waveform and the expiratory flow waveform. For PC, this is recognized by observing the flow waveform. There should be no evidence early or late cycle. Sometimes a sharp rise in end-inspiratory pressure can be a ventilator artifact.25
Late Cycle
Inspiration ends with a clinically important delay after the Pmus peak. Another way to state this is that the patient’s neural inspiratory time is shorter than the inspiratory time imposed by the ventilator. It can also be observed with spontaneous breaths if the flow cycle threshold is set too low or when patient effort ceases but it fails to cycle inspiration. The primary example is the “runaway” phenomena in the mode called PAV, where the ventilator continues the assistance in spite of patient terminating inspiratory effort due to the ventilator’s inaccurate estimation of lung mechanics.
Late cycling must be assessed according to the clinical context. For example, if a patient makes only a short trigger effort and inspiration is time or volume cycled, then by definition this is late cycling. It may be perfectly acceptable from both a patient safety and comfort point of view. However, late cycling becomes relevant when there is evidence of expiratory effort before the cycle event. In VC, the pressure waveform increases abruptly at end inspiration, indicating expiratory effort or respiratory muscle relaxation. The same can sometimes be seen with PC, and in addition, the expiratory effort will cause a downward deflection in the flow waveform, possibly even crossing into negative flow before the ventilator cycles inspiration (Figure 11).26
Early Cycle
Inspiration ends within clinically important time before the Pmus peak. The cycle event occurs before the patient effort ceases. Another way to say this is that the patient’s neural inspiratory time is longer than the inspiratory time imposed by the ventilator (Figure 11). For both VC and PC, this is recognized in the expiratory flow waveform as a distortion of the peak expiratory flow and disruption of the normally smooth exponential flow decay of passive expiration. Early cycle is a common cause of multiple triggering (also known as double trigger). With PC, in some patients with very prolonged time constants (eg, COPD, asthma), flow may still be positive at the end of the inspiratory time. However, if there is no evidence of inspiratory effort during the early expiratory phase, this is not a synchrony problem.
Early cycle can also occur if the ventilator cycles inspiration by a non-patient signal. This is typically recognized as an unusually short inspiratory time; for example, a patient with very low compliance and a rapid rise time in pressure support mode. This causes a high peak flow followed by rapid decay due to a short time constant, thereby too rapidly reaching the flow cycle threshold. It can also occur if ventilator safety features, such as a pressure limit or a spontaneous tidal volume limit, are reached.
Expiration
During expiration (ie, the period from the start of negative flow to the start of positive flow), the patient-ventilation interaction is not characterized by timing (synchrony) but by work. Normal expiration is passive. During expiration, the ventilator controls the pressure with set-point targeting (ie, the target value is PEEP). In a passive patient, we expect to see a smooth exponential decay of the expiratory flow and volume waveforms. This allows us to observe the predominant physiology and the effects of trigger and cycle discordances. The expiratory phase can demonstrate, as described above, other patient-ventilator interactions (early cycle and failed trigger); however, the one that is proper to the expiratory phase is expiratory work.
Expiratory Work
Patient expiratory effort (ie, negative Pmus) will deform the flow waveform in a negative direction (away from baseline). Expiratory work may be normal, as when exercising or coughing, but it may also indicate the presence of high resistive load (eg, COPD), acidosis, or anxiety (Fig. 4).
Waveform Patterns
Ventilator waveform patterns are important to recognize as they may have clinical implications. A waveform pattern is a sequence of or stereotypical waveforms that may have a clinical consequence and are due to a patient-ventilator interaction. Many reports describe these patterns as a specific patient-ventilator interaction; however, as described below, they can have several etiologies. We approach these separately from the standardized waveform analysis. Patterns should trigger further evaluation.
Multiple Trigger
Multiple trigger is characterized by 2 or more ventilator breaths delivered in close succession without complete expiration between them. Many ventilators have a trigger window immediately after inspiration where another breath can’t be triggered; this leads to a brief but consistent pause between mechanical inspirations. Terminology for this phenomenon varies in the literature. It is sometimes called double triggering, double cycling, clusters, or breath-stacking. All these terms have issues with meaning or accuracy (see related supplementary material at http://rc.rcjournal.com). Although double trigger is the most common presentation, there are instances where multiple breaths are triggered, and thus the term multiple. Multiple trigger is a pattern that has at least 3 causes (Figure 12): early cycle, early trigger, and false trigger.27 Its presence has been associated with poor outcomes,28 the main concern being, in VC modes, overdosing of tidal volume by breath-stacking leading to ventilator-induced lung injury (this is less so in PC, as VT is dependent on R and C).
Causes of multiple trigger. A. Early trigger, the pattern is mandatory (machine triggered) followed by evidence of Pmus, and then a patient triggered breath. B. False trigger, no evidence of Pmus and triggering of a breath immediately after exhalation. C–D. Early cycle: evidence of Pmus through first breath leading to triggering of next breath. Note: red Pmus line was overlaid by hand onto a ventilator screen image, and Pmus is shown inverted for clarity.
Tidal Volume Discrepancies
The volume waveform contains mostly the same information as the flow waveform; however, there is one area where it conveys specific information. Knowledge of how the ventilator displays volume is key. The volume waveform is derived from the flow measurements for most ventilators; volume is the integral of flow, which means that the area under the flow curve over the inspiratory flow time equals the tidal volume. At the beginning of each breath (patient or machine triggered), the ventilator resets the volume waveform to zero so that the inspiratory tidal volume displayed is accurate. This means that differences in inhaled and exhaled tidal volumes manifest with a pattern where the volume waveform has a sharp drop (reset) prior to the next breath, similar to a square root sign (Figure 13). This pattern has at least 4 causes: (1) leak from the circuit, airway, or lung; (2) active exhalation during inspiration (some ventilators do not account for VT exhaled during inspiration)26; (3) air trapping, the patient has not been able to exhale the inhaled VT, and another breath is triggered; and (4) flow sensor malfunction.
There are many etiologies that exist for inspiratory and expiratory discrepancy. This example is due to a leak (around endotracheal tube) causing false trigger. The ventilator flow trigger detects inspiratory flow (due to the leak) and triggers a breath. Note the characteristic “volumen reset” for the new breath leading to the “square root sign.”
There are multiple other artifacts and perhaps other patterns. This manuscript does not intend to be an exhaustive review of all possible patient-ventilator interactions. Instead, we offer a standard nomenclature and an organized method to read waveforms from which we are sure much will be added, researched, and improved.
Applying the Method
We have created a tool to aid the clinician in systematically reading waveforms (Table 4). This method also allows one to summarize the patient state in a single sentence (eg, The patient is on PC-CMVa, with a high elastic load and has early triggers). It is common that more than one discordance is found in a given tracing. Some discordances are associated with others (eg, early trigger is commonly followed by early cycle and work shifting), in which case we only mention the first discordance (ie, early trigger). Following this method will allow the clinician to decide if that patient-ventilator interaction matches the goals of ventilation and if it does not to determine the changes in settings or modes needed to achieve the goals.
Systematic ventilator waveform analysis tool
In our practice, we emphasize that it is easier to decide what to do when you know what the goal is. There are only 3 goals of mechanical ventilation (safety, comfort, and liberation).7 They are not mutually exclusive, but one must choose which is primary at the given time. For example, in a patient with ARDS in the first days, the main goal is safety. We want to ensure the settings and interactions lead to lung-protective ventilation and ensure gas exchange. Comfort is important, but we would not choose a mode that favors comfort over lung protection. As the patient recovers, the clinician is likely to be attempting liberation; yet if the patient is not ready, comfort (improving synchrony and work of breathing) in order to minimize sedation would be the leading goal. Safety (ie, preventing lung injury and ensuring gas exchange) is still important, but modes that serve comfort and yet maintain safety should be preferred.7
This also helps put the interpretation of the waveform in context, as the changes done to the ventilator should be in line with the goal of mechanical ventilation. For example, a patient with extreme work shifting, in the setting of safety as a goal, will guide the clinician toward correcting the cause (eg, sedation, neuromuscular blockade, correction of metabolic acidosis).
A word of caution to the reader, patient-ventilator interactions occur breath by breath; and as such, they will change with patient condition, level of awareness, interventions, etc. Many interactions will be temporary, harmless, and may be irrelevant clinically (eg, mild work shifting); others may be harmful, especially if frequent (eg, multiple trigger in VC). Not every interaction requires an intervention.
Conclusions
Modern ICU ventilator displays provide a complex array of information on the patient’s condition. A standard nomenclature is needed to ensure we communicate our findings clearly and consistently. A systematic approach is needed to ensure consistent diagnosis and treatment for patient-ventilation interaction issues and ultimately improve outcomes. The methodical evaluation of the waveform provides information that can lead to changes in how we implement an intervention that has the ability to save lives but also cause harm. Our proposal delivers a method and taxonomy based on prior published work to help clinicians achieve these goals.
Footnotes
- Correspondence: Eduardo Mireles-Cabodevila MD. E-mail: Mirelee{at}ccf.org
Dr Mireles-Cabodevila is a co-owner of a patent for mid-frequency ventilation. He discloses relationships with American College of Physicians and Jones & Bartlett publishers. Mr Chatburn discloses relationships with IngMar Medical, Vyaire Medical. He is a co-owner of a patent for mid-frequency ventilation. Dr Siuba has disclosed no conflicts of interest.
Supplementary material related to this paper is available at http://rc.rcjournal.com
- Copyright © 2022 by Daedalus Enterprises