In this issue of Respiratory Care, Vargas et al present the results of a randomized study comparing neurally-adjusted ventilatory assist (NAVA) versus variable pressure support (VPS) ventilation.1 The goal was to evaluate the effect of both modes on synchrony and work of breathing. The study was carefully planned, and a fair amount of information is given for us to review and compare. At the end, no major differences were found. To the reader, this may seem as just another trial with evidence of no difference. However, there are many details, based on how the modes work and are tested, that can give perspective to their results.
A mode of ventilation is a predetermined pattern of patient-ventilator interaction that can be classified by 3 characteristics: (1) control variable, that is, pressure or volume; (2) breath sequence, that is, continuous mandatory ventilation, intermittent mandatory ventilation, or continuous spontaneous ventilation; and (3) targeting scheme(s).2 Such taxonomy is important for distinguishing arbitrary brand names from generic classifications, just as is done in pharmacology. Once taxonomy aspects are standardized, it is easier to evaluate performance in terms of clinical outcomes (eg, mortality), technological features serving the goals of ventilation (ie, safety, comfort, and liberation),3 patient-ventilator interaction (ie, synchrony issues),4 and/or discrete physiological outcomes (eg, work of breathing). Vargas et al1 focused on compared physiological outcomes (synchrony and work of breathing) between NAVA and VPS. Their study allows us to discuss a framework to compare modes. Evidently, modes differ substantially with respect to technical capabilities as well as settings used to manage them. Thus, a systematic comparison of mode characteristics could help understand differences and potential sources of bias when assessing performance. Table 1 summarizes a construct to assess the differences between NAVA and VPS with pressure support (PS) added as a comparator.
Technical Comparison of Modes
The first insight is that even though these modes control inspiratory pressure and have the same breath sequence, the targeting scheme (ie, feedback control software) is different. NAVA uses servo targetting and VPS biovariable targeting. NAVA uses the diaphragm electrical activity (EAdi) to control the timing and magnitude of the pressure delivered. The pressure delivered in NAVA is proportional to the amplitude of the EAdi signal, and the proportionality is regulated by the set NAVA level (cm H2O/mV of EAdi). VPS is similar to PS in the way we set trigger, cycle and inspiratory pressure, and the average or mean PS. However, in VPS, the operator sets also the variability of the inspiratory pressure as a percentage (0–100%) of the target inspiratory pressure. The delivered inspiratory pressure values are defined randomly, but follow a gaussian distribution (extreme values are rare, whereas values around the mean occur more frequently). For example, a setting of 100% means that the inspiratory pressure for any given breath varies randomly from 100% below to 100% above (double) the set inspiratory pressure target. If the maximum support achievable is limited by the maximum pressure setting, the variability is reduced in order to not exceed that limit, while keeping a gaussian distribution of PS levels. Currently, NAVA is available only in the Getinge Servo ventilator, whereas VPS is available only in the Dräger ventilator V500. This is important because the programming and performance may change if other ventilator platforms develop or if there are updates to the ventilator software.
Table 1 helps highlight potential differences in how the ventilators may perform when evaluated. For instance, when evaluating trigger performance, NAVA uses the EAdi signal that leads (ie, occurs before) the airway pressure and flow signals (Fig. 1) that trigger and cycle inspiration for PS and VPS. The method to set the trigger and cycle thresholds for each mode may lead to systematic differences that can be ascribed to the operator rather than the mode per se. In the study of Vargas et al,1 an arbitrary fixed trigger threshold (2 L/min) was used for VPS rather than the maximum sensitivity that was not associated with false triggering.5 Although there was no difference noted in this study, in theory, this may bias measurements of trigger delay and trigger work against VPS. Although this potential bias might be negligible from a clinical standpoint, differences may yield conclusions with clinical relevance, such as choosing one mode over the other.
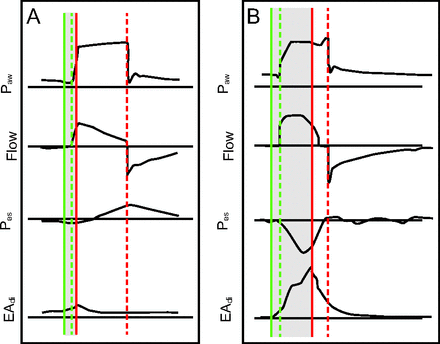
Neuromechanical uncoupling and its effects on patient ventilator synchrony. EAdi = diaphragm electrical activity. Pes = esophageal pressure. Paw = airway pressure.
All breaths are pressure control. Green solid line is start of neural signal; green dashed line: start of inspiratory flow from ventilator (trigger event); red solid line: peak of the EAdi (end of neural time); red dashed line: end of inspiratory flow from ventilator (cycle event); blue dashed lines: difference between maximum EAdi and minimum Pes. Shaded area: period of neural inspiratory time. A: Patient with overassistance. EAdi has low amplitude and short duration. Note delay in trigger event compared to start of EAdi signal. Note also the delay in cycle event well after peak EAdi. Airway pressure is out of synchrony with EAdi. B: Patient with underassistance. There is a similar trigger delay as in A, but the cycle delay is much less. However, in this case delayed cycling is evident by rise in end-inspiratory pressure as the ventilatory muscles relax.20 Note the dissociation of the maximum EAdi and minimum Pes, consistent with neuromechanical uncoupling. Modified from Reference 8 and Reference 21.
With regard to inspiratory pressure, NAVA and VPS have different targeting schemes. In the study of Vargas et al,11 the NAVA level and the set inspiratory pressure in VPS were adjusted to achieve a tidal volume of 6–8 mL kg. However, for VPS, the inspiratory pressure variability was set to 100%, which means that the patient will get a large range of inspiratory pressures around the set mean inspiratory pressure. From a synchrony standpoint, the larger variability range may predispose VPS to both underassistance and overassistance6 of some breaths. Overassistance might explain the significantly higher incidence of failed triggers in the study of Vargas et al.1 On the other hand, the large range of pressure variation may also have a protective effect against continuous overassistance.
The cycling criterion is also relevant. NAVA uses a fixed threshold (70% of the peak EAdi), whereas in VPS the threshold can be adjusted. Vargas et al chose to use a fixed threshold of 25% of the peak flow.1 Although these fixed settings may lead to fair comparisons, it can also explain the nonsignificant but higher incidence of delayed cycle for VPS. In terms of NAVA, the fixed threshold is a known cause of double trigger6 and has been frequently reported.7-11 This seems to be a technical artifact due to the fixed cycle threshold in the setting of irregular EAdi activation. The waveform meets the definition criteria used in most trials; that is, a double trigger is 2 breaths (2 or more triggers leading to pressurization) separated by an expiratory time less than half the mean inspiratory time.4,12 However, not all double-trigger events are the same. Consider the double trigger that occurs in volume control versus pressure control. In volume control, the patient will receive double the tidal volume (colloquially referred as breath-stacking), which can lead to volutrauma. In pressure control, the patient will receive the second breath at the set pressure for the set inspiratory time, and the tidal volume will depend on the respiratory system compliance and resistance as well as the pressure generated by the ventilatory muscles. The risk of volutrauma is lower; however, the theoretical risk for diaphragm injury via eccentric contractions increases. In PS, double trigger will lead to repressurization of the airway that will cycle off again per usual criteria, the effects of which are unknown but likely involve less risk of volutrauma and diaphragm injury. In NAVA, double trigger leads to brief pressurizations and volume change, the effects of which are unknown, but likely similar to PS. These differences are not accounted by definitions or by lumped indexes (more on this below).
The definitions and methods used to evaluate mode performance (in terms of esophageal pressure [Pes], EAdi, or waveform analysis) may lead us to favor one mode over another.13 For example, the way we measure and define synchrony. Both the EAdi and the Pes have been heralded as the accepted standard for evaluation of patient-ventilator interactions. Both are invasive and provide relevant information regarding timing and magnitude of the effort. However, the presence of neuromechanical uncoupling may affect the interpretation of the interactions when using Pes versus EAdi. Neuromechanical uncoupling refers to the difference between neural signal and the mechanical response (change in airway flow/pressure) of the respiratory system. In Figure 1, one can note the discrepancies that would occur if the Pes or the EAdi was used to measure the discordances. Importantly, the Pes signals will not detect uncoupling events, which can be relevant in how we classify interactions.8,14 Neuromechanical uncoupling has several clinically important causes such as air flow obstruction and overassistance. The science of patient-ventilator interaction is evolving, and we need to define a set of definitions and standards for measurement and reporting of synchrony that accounts for these nuances.
Finally, we often compare modes using measures of global synchrony, such as the asynchrony index. Such an index may be calculated in different ways; some use the EAdi,15,16 others the Pes,17 and others waveform analysis.18 In all these cases, the same threshold of 10% is used, which likely has different performance across methods. The type of patient-ventilator discordance included in the global index will also affect comparisons (eg, including discordances that deal with work/demand vs those that involve timing). By lumping all discordances together, we lose precision in comparing modes. Some synchrony issues are clinically irrelevant and may simply be an analytical distraction. Some may be due to inappropriate settings. Other relevant measures, such as overassistance or early trigger (also known as reverse trigger), are not included. Any asynchrony index needs to be taken with these caveats in mind. In the study of Vargas et al,1 modes did not differ significantly with respect to the asynchrony index, yet we can see that many of the interactions that influenced the incidence of the global index were perhaps due to the settings and to technical issues other than mode performance per se.
Patient-ventilator synchrony is a commonly evaluated outcome in mechanical ventilation. It is a flourishing area of research and clinical application. Based on recent studies, it might also affect clinical outcomes.19 It follows that our assessment of synchrony needs to be reliable and clear. At this time, we lack a unified reporting taxonomy for patient-ventilator interactions; we lack clarity on which tools to use to assess patient-ventilator interactions, and finally we lack of a standardized method to apply measuring tools. As we work to advance our understanding of mechanical ventilation, synchrony, and work of breathing, a unified multisociety consensus would be welcome. This would allow thoughtful studies such as this by Vargas et al1 to be even more useful.
Footnotes
See the Original Study on Page 503
Dr Mireles-Cabodevila is a co-owner of a patent for mid-frequency ventilation. He discloses relationships with the American College of Physicians, Elsevier, and Jones & Bartlett publishers. Dr Gama de Abreu was granted a patent on variable pressure support (VPS) ventilation that is licensed to Dräger Medical. He discloses relationships with Ambu, ZOLL, Lungpacer, GE Healthcare, Dräger Medical, and Novalung.
- Copyright © 2022 by Daedalus Enterprises