Abstract
BACKGROUND: The effect of the respiratory therapist (RT)/patient ratio and RT organizational factors on respiratory resource utilization is unknown. We describe the impact of a multi-component intervention that called for an increase in RT/patient ratio (1:14 to 1:10), improved RT orientation, and formation of a core staffing model on best practice, including spontaneous breathing trials (SBTs) and catheter and bronchoscopically directed lower respiratory tract cultures, or bronchoalveolar lavage (BAL), in both ventilated and non-ventilated patients in the ICU.
METHODS: We conducted a single center, quasi-experimental study comparing 651 patients with single and first admissions between April 19, 2005 and April 18, 2006 before the RT services reorganization with 1,073 patients with single and first admissions between September 16, 2007 and September 4, 2008. Baseline characteristics were compared, along with SBTs, BAL use, lower respiratory tract cultures, and chest physiotherapy.
RESULTS: Patients in the 2 groups were similar in terms of age (52.9 ± 15.8 y vs 53.9 ± 16.4 y, P = .23), comorbidity as measured by Charlson score (2.8 ± 2.6 vs 2.8 ± 2.7, P = .56), and acuity of illness as measured by the Case Mix Index (3.2 ± 3.9 vs 3.3 ± 4.1, P = .47). Mechanically ventilated patients had similar prevalences of respiratory diseases (24.2% vs 25.1%, P = .61). There was an increase in SBTs (0.5% vs 73.1%, P < .001), chest physiotherapy (7.4% vs 21.6%, P < .001), BALs (24.0% vs 41.4%, P < .001), and lower respiratory tract cultures (21.5% vs 38.0%, P < .001) in mechanically ventilated patients post-intervention.
CONCLUSIONS: A multi-component intervention, including an increase in RT/patient ratio, improved RT orientation, and establishment of a core staffing model, was associated with increased respiratory resource utilization and evidence-based practice, specifically BALs and SBTs.
- personnel staffing and scheduling
- critical care
- respiratory care units
- mechanical ventilators
- health resources
- work load
Introduction
One of the defining characteristics of medical critical care is the demand for respiratory care services and the care of patients with respiratory failure. Respiratory care, particularly of mechanically ventilated patients, is responsible for a large proportion of resource utilization in the ICU.1,2 Mechanical ventilation is associated with life-threatening complications, including volutrauma, barotrauma, and ventilator-associated pneumonia (VAP).3 These complications lead to increased costs and mortality.3,4 Not surprisingly, ventilator liberation has been the focus of much research.
Current evidence has demonstrated that protocols and procedures driven by respiratory therapists (RTs) improve outcomes.5 RTs are healthcare professionals whose responsibilities include diagnostic evaluation, management, education, assessment, and rehabilitation of patients with diseases of the cardiopulmonary system.6 RT-initiated weaning protocols lead to earlier ventilator liberation, compared with physician-directed methods.7–9 Current guidelines support the use of lower respiratory tract cultures in the diagnosis of VAP.10 Obtaining these cultures typically requires the assistance of RTs for flexible, fiberoptic bronchoscopic bronchoalveolar lavage (B-BAL) or non-bronchoscopic, catheter-directed BAL (N-BAL). While RTs play an important role in the management of mechanically ventilated patients, few studies have aimed to establish an optimal number of RTs per patient (RT/patient ratio) or examine the impact of an improved RT training curriculum on resource utilization. Currently, no well established evidence-based guidelines exist for RT staffing in the ICU. The Society for Critical Care Medicine recommends that “an appropriate number of respiratory therapists with specialized training must be available to the unit at all times.”11
In a prior study evaluating this cohort and utilizing the same data, we found that a multi-component reorganization of medical ICU resources, including changes in RT staffing and orientation, was associated with a reduction in mortality and an increase in 28-day ventilator free days.12 The reorganization of respiratory care services included an increase in the RT/patient ratio, improved RT orientation and education, and the formation of a core staffing model. In assessing potential mechanisms for this improvement in outcomes, we hypothesized that this intervention was associated with increased utilization of best-practice procedures, including spontaneous breathing trials (SBTs) and BALs (bronchoscopic and non-bronchoscopic) in both ventilated and non-ventilated patients in the ICU. To our knowledge, we are the first to study the impact of an increase in RT/patient ratio, improved training curriculum, and formation of a core staffing model on respiratory care resource utilization and evidence-based practices in the ICU.
QUICK LOOK
Current knowledge
The impact of the respiratory therapist-to-patient ratio and organizational factors on respiratory care resource utilization and patient outcomes is unknown. Respiratory therapists can impact patient outcomes with evidence-based practices, including assessments of weaning readiness and implementation of ventilator-associated pneumonia prevention bundles.
What this paper contributes to our knowledge
An increase in respiratory therapist-to-patient ratio in the ICU, from 1:14 to 1:10, coupled with a core staffing model and intensive orientation program, increased the utilization of daily spontaneous breathing trials, collection of lower respiratory tract cultures for diagnosis of ventilator-associated pneumonia, chest physical therapy, and inhaled nitric oxide. The cumulative impact on patient costs and outcomes was not assessed.
Methods
Environment and Intervention
We conducted a single-center, quasi-experimental study13,14 of all patients admitted to the University of Maryland Medical Center medical ICU (MICU) between April 19, 2005, and April 18, 2006 (12 months pre-intervention) and September 16, 2007, and September 4, 2008 (12 months post-intervention). The University of Maryland Medical Center is a 745-bed urban teaching hospital. Prior to the intervention there were 705 total beds. These dates reflect one-year periods before and after the staggered implementation of the multi-step intervention, which occurred between April 19, 2006, and September 15, 2006.
Pre-intervention, the RT department was staffed by a combination of University of Maryland Medical Center employees and locum tenens RTs. There were 10 beds in the pre-intervention MICU, and the RT/patient ratio varied between 1:14 and 1:19. The RTs pre-intervention were often responsible for patients in the medical intermediate care unit as well as other ICUs. The multi-component intervention consisted of a physical move to a new, larger MICU, the creation of a core-RT model, and an enhanced RT training program. The decision to expand the ICU was made in response to increased demand for medical critical care and as a part of the move to a new hospital building, and was unrelated to prior clinical performance of the ICU. The rationale for the RT intervention was to provide adequate staffing for the new MICU and to reduce staffing turnover. The new 29-bed MICU is staffed by at least 3 RTs at all times, with a resultant RT/patient ratio of no less than 1:10. The hiring of locum tenens RTs ceased after the move. In addition, the core-RT model was implemented on September 16, 2007. This model requires 3 RTs to be present on both day and night shifts. At least one registered RT (core RT) with a chosen clinical concentration in medical, as opposed to surgical or trauma, intensive care is on the unit at all times. In 2006 the new hire orientation program for RTs was also modified. Prior to this change, orientation included accompanying a veteran RT for several days before working independently. In June 2006 a classroom-style orientation was implemented, consisting of a full-time 4-week lecture series. All procedures and pieces of equipment are reviewed, and a competency exam is conducted after the course. The lectures are taught by a multidisciplinary team comprised of RT clinical coordinators, nurse educators, physicians, and vendors. Following this classroom orientation, the new RTs rotate through 12-hour shifts in more than 3 different ICUs with a preceptor. The new hires are paired with a core RT for the first 6 months. This orientation process occurs 2–3 times annually, with classes ranging in size from 6 to 19 RTs. There was a change in RT administrators at the time of our intervention. The reorganization of respiratory therapy in the ICU was performed in collaboration with an external consultant (DH).
These changes in respiratory care were part of a multidisciplinary intervention that also included the institution of 24-hour intensivist coverage and the addition of a pharmacist to the multidisciplinary team.15 These changes are shown in Figure 1. The RTs' scope of practice, including the procedures performed, did not change. Computerized order reconciliation, including a respiratory care pathway, was implemented after 2006. The medical director of the MICU did not change. In addition to the stability of nursing staffing ratios over this time period, the MICU nursing leadership remained unchanged. There was no change in laboratory, radiology, or consultant services after the intervention, which continued to be fully available 24 hours daily. No changes in protocol or educational campaigns for diagnostic procedures for pneumonia were introduced during the entire study period. Both pre- and post-intervention, B-BALs were obtained by physicians with RT assistance, while N-BALs were performed by RTs, independent of physicians. Similarly, the SBT protocol did not change before or after the intervention, though a computer order reconciliation program was introduced after the intervention.
Timeline of interventions surrounding the move to the new medical ICU (MICU) and reorganization of respiratory care.
This study was approved with waiver of consent by the University of Maryland institutional review board.
Data Sources and Patient Selection
Our primary outcome variables were the number of B-BALs, N-BALs, SBTs, upper respiratory tract cultures, and lower respiratory tract cultures. Data were abstracted from the hospital's electronic medical record and administrative record systems, maintained by the Information Technology Group of the University of Maryland Medical Center as a clinical data repository. The clinical data repository is a relational database that has microbiology, pharmacy, admission/discharge/transfer, pathology, and radiology data. The pharmacy, microbiology, and medical demographics tables in the relational database have been regularly validated against medical records in more than 1,400 patients admitted between October 1997 and July 2008. In prior studies, the positive and negative predictive values of random 10% sampling of the data were > 99% accurate, when compared to patients' medical charts.16–21 A 10% random sample of the administrative data recording ventilator use found an accuracy rate of 94.1% (1,941/2,062 patient days). Financial data were abstracted from the medical center's administrative data.
We evaluated 3,513 consecutive patients in the 24 months pre-intervention and 24 months post-intervention with first and single admissions on the data file, to protect independence of observations and, to a lesser extent, comparability of the clinical courses studied. Patients missing All Patient Refined Diagnosis-Related Group or International Classification of Diseases, 9th Revision, Clinical Modification codes were excluded from analysis, as were patients with coded negative lengths of stay. We excluded patients admitted during a transition period during which the RT/patient ratio varied.
Analytic Approach
We evaluated baseline characteristics of the 2 groups (pre and post intervention), including age, sex, race, percent mechanically ventilated, primary diagnosis, comorbidity as expressed by the Charlson score, and severity of illness as measured by the Case Mix Index weight, a Maryland state measure based on diagnoses and used to adjust hospital reimbursement for expected resource intensity of treatment. (Case Mix Index scores are weighted to a state average of 1.00.22) The baseline MICU admission characteristics pre- and post-intervention were compared using 2-sample t tests for continuous variables,23 and comparisons of proportions for categorical variables.24 The total units performed, the proportion of patients receiving them, and the mean number of units per patient before and after intervention were compared with 2-sample t tests and Mann-Whitney tests.25
RT services utilization was evaluated among all patients meeting study criteria. The following services were identified a priori for analysis: BAL (bronchoscopic and catheter-directed), lower respiratory tract cultures, nasal washings, mechanical and manual chest physiotherapy, incentive spirometry, SBTs, endotracheal tube/tracheostomy care, arterial blood gas sampling, vital capacity, noninvasive ventilation, nebulizers/inhalers, and inhaled nitric oxide. Because mechanically ventilated patients have greater respiratory therapy needs, we performed additional analyses of patients who received one or more days of mechanical ventilation, and patients who did not receive mechanical ventilation, to determine if there was a difference in resource utilization between the 2 groups post-intervention. In order to control for changes in the incidence of VAP when examining BALs, N-BALs, and culture data, the proportion of patients with any respiratory culture (both upper and lower) was examined pre- and post-intervention. Analyses were performed using statistics software (SAS 9.1.3, SAS Institute, Cary, North Carolina). We applied the traditional definition (P ≤ .05) of statistical significance.26
Results
There were 651 patients pre-intervention and 1,073 patients post-intervention for analysis (Fig. 2). Thirty patients were excluded for missing All Patient Refined Diagnosis-Related Group codes and International Classification of Diseases, 9th Revision codes. Patients admitted after the intervention were similar to those admitted pre-intervention (Table 1). There were no differences in age, sex, race, proportion of patients receiving one or more days of mechanical ventilation, comorbidity as assessed by the Charlson score, or acuity of illness as assessed by the Case Mix Index score. There were no significant changes in the primary diagnoses of patients, though there was a small increase in circulatory-related and neoplasm admissions and corresponding modest declines in the relative incidence of other diagnoses. After the intervention there was an increase in the number of procedures and the proportion of patients undergoing B-BAL, N-BAL, diagnostic nasopharyngeal washing, chest physiotherapy, and incentive spirometry (Table 2, Fig. 3).
Enrollment of study participants. MICU = medical ICU. APR-DRG = All Patient Refined Diagnosis-Related Group. ICD-9-CM International Classification of Diseases, 9th Revision, Clinical Modification.
Subject Characteristics
Respiratory Therapy Use in All Patients
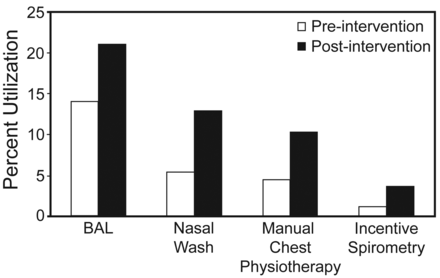
Percent respiratory therapy resource utilization in all patients (mechanically ventilated and non-ventilated) pre- and post-intervention. BAL = both bronchoscopic and non-bronchoscopic, catheter-directed BAL. Nasal wash = nasal sampling for respiratory viruses.
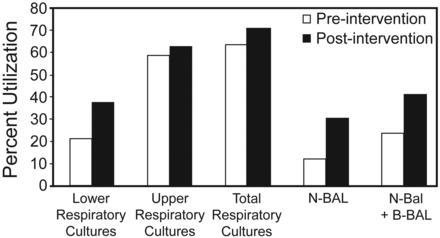
Among patients receiving one or more days of mechanical ventilation, there were no differences in age, sex, race, Charlson score, Case Mix Index weight, or primary diagnosis (see Table 1). A greater proportion of these patients received chest physiotherapy, incentive spirometry, endotracheal tube or tracheostomy care, arterial blood gas puncture, SBTs, nitric oxide therapy and measurement of vital capacity (Table 3, Fig. 4). The proportion of patients undergoing lower respiratory tract diagnostic procedures (ie, B-BAL or N-BAL) and the number of procedures per patient increased after the intervention (Fig. 5). In order to demonstrate that this increase was not due to an increased incidence of VAP or suspected VAP, the proportion of patients with any respiratory culture (both upper and lower) was used as a control and examined pre- and post-intervention. The proportion of lower respiratory tract cultures to total respiratory cultures was assessed for pre- and post-intervention patients. Post-intervention, an increased proportion of patients underwent lower respiratory tract diagnostic procedures (24.0% pre-intervention vs 41.4% post-intervention, P < .001) and had lower respiratory tract specimens obtained (21.5% vs 38.0%, P < .001). The proportion of respiratory cultures that were obtained from the lower respiratory tree increased after the intervention (18.6 ± 30.9% vs 30.1 ± 34.3%, P < .001). There was no difference in the proportion of patients undergoing upper respiratory tract cultures before and after the intervention (59.5% vs 63.4%, P = .26).
Respiratory Therapy Use in Mechanically Ventilated Patients
Percent respiratory therapy resource utilization in mechanically ventilated patients pre- and post-intervention. SBT = spontaneous breathing trial. Nasal wash = nasal sampling for respiratory viruses.
Percent utilization of respiratory culture resources in mechanically ventilated patients pre- and post-intervention. N-BAL = non-bronchoscopic, catheter-directed bronchoalveolar lavage. B-BAL = bronchoscopic BAL.
Among patients who were never mechanically ventilated, there were no differences between the pre- and post-intervention groups with regards to age, race, sex, comorbidity as measured by the Charlson Score, or illness severity as demonstrated by the Case Mix Index (see Table 1). There were significant differences between the 2 groups in terms of MICU primary diagnosis (P = .01). There were no differences in respiratory therapy resource utilization between non-ventilated patients before and after the intervention with regards to: nebulizers, B-BALs, upper respiratory tract cultures, nasal washings, mechanical chest physiotherapy, incentive spirometry, and noninvasive mechanical ventilation (Table 4). A greater proportion of patients who were never mechanically ventilated had arterial blood gas sampling after the intervention (17.2% vs 23.4%, P = .03).
Respiratory Therapy Use in Non-ventilated Patients
Discussion
Respiratory care plays a central role in the management of patients with critical illness. Our study found increased utilization of respiratory resources, including B-BALs, N- BALs, and SBTs, and greater frequency of evidence-based practices occurred after a multi-component intervention that called for improved RT staffing (including elimination of locum tenens RTs) and orientation. Determination of the degree to which each individual component contributed to the results observed is beyond the scope of this study. To our knowledge, this is the first study to examine respiratory resource utilization and adherence to evidence-based practice after changes in respiratory staffing and orientation. These improvements in care point to a potential mechanism for our prior findings of a reduction in the odds ratio of death by 22% (95% CI 0.61–0.99, P = .04) and increased 28-day ventilator-free days (median 21 d [IQR 0–25 d] vs 22 [IQR 0–26 d], P = .04) among mechanically ventilated patients after a multi-component intervention.
Currently, no concrete guidelines exist regarding RT staffing ratios. A 2006 study, surveying RTs at 30 hospitals ranging in bed size from < 250 to > 500 beds, concluded that increased RT staffing would be necessary if ICUs continued to grow as projected.27 A recommendation was made that one new RT be hired for every 11.3 beds added to an ICU, in order to maintain appropriate patient care.27 Our study found that an improved RT staffing ratio of 1:10 (increased from 1:14) was associated with increased utilization of best-evidence practice in the care of mechanically ventilated patients.
The current literature shows the importance of SBTs in the management of mechanically ventilated patients. Current guidelines recommend the use of SBTs to determine which patients are appropriate for ventilator liberation.28 Ventilator liberation protocols that incorporate daily SBTs have been shown to increase ventilator-free days and decrease hospital stay, compared to physician-driven ventilator liberation.29–33 Daily SBTs in mechanically ventilated patients lead to decreased duration of ventilation as well as fewer complications, when compared to patients who did not undergo an SBT.29 SBTs have also been associated with earlier extubation than trials of pressure support or intermittent mandatory ventilation,34 and protocol-driven SBTs increase the extubation rate without a change in reintubation rates, when compared with unregulated ventilator management.33 The Awakening and Breathing Controlled Trial showed that protocols pairing interruption of sedation with daily SBTs improved outcomes in ICU patients.35,36 While any non-physician healthcare provider was permitted to utilize weaning protocols in many of the above studies, there are several trials showing the same benefit with regard to outcomes when the protocols were implemented by RTs only.7,32 At our study institution, SBT protocols are implemented exclusively by RTs. Our study shows that improved RT training and staffing increased adherence to daily SBTs.
This multi-component intervention was associated with increased utilization of lower respiratory tract cultures. While controversy exists regarding the diagnosis and management of VAP, current American Thoracic Society guidelines favor the use of lower respiratory tract cultures in diagnosing and managing VAP.10 Lower respiratory tract cultures, when used as part of a clinical scoring system, decrease costs and reduce antibiotic usage in the management of VAP,37 and facilitate early appropriate antibiotic use in patients suspected of having VAP.38 A randomized controlled trial demonstrated improved outcomes, including a decrease in mortality and less antibiotic use, when BAL was used as part of the clinical diagnostic criteria for VAP, as opposed to endotracheal aspirates alone.39 At our study site, RTs perform catheter-directed BALs and assist with bronchoscopic BALs, which are the preferred methods of obtaining lower respiratory tract cultures. Our study shows an association with improved RT staffing and evidence-based practice in the diagnosis and management of patients with VAP.
RTs are an essential part of the multidisciplinary team. Daily rounds by a multidisciplinary care team are associated with lower mortality.40 Implementation of a 24-hour in-house attending is associated with improved outcomes in the ICU.41 It is likely that the benefit of increased and reorganized RT staffing in our ICU was maximized by the structure of our MICU, which is characterized by both full multidisciplinary staffing and rounds, and around-the-clock intensivist coverage. For example, while both B-BAL and N-BAL are ordered by physicians, we hypothesize that the availability and collaboration of RTs in patient care facilitated the ordering and completion of these diagnostic procedures.
Given our study's quasi-experimental design, we took care to assess whether or not the increase in lower respiratory tract cultures post-intervention was not due simply to a change in case mix or an increase in the incidence of VAP. We evaluated both the total number of lower respiratory tract cultures obtained, and the proportion of respiratory tract cultures (upper vs lower) as a control. We observed an increase in the total number of cultures occurring after the intervention. Of these, lower respiratory tract cultures accounted for the greater proportion of culture type, consistent with a change in practice independent of possible temporal changes. While no reciprocal decrease in upper respiratory tract culture sampling was found, we are confident that there was an increase in use of lower respiratory tract cultures, which has been shown to improve VAP treatment. Previous analysis of the MICU patient population pre- and post-intervention showed that the case mix remained stable over the entire period studied.12
Our study does have certain limitations. Although we found no difference between the 2 groups with regard to illness severity and comorbidities using the Case Mix Index and Charlson score, respectively, and no difference between the 2 groups of mechanically ventilated patients with regard to primary diagnosis, residual confounding may still have occurred, which is possible in quasi-experimental studies. A large proportion of the data assessing ICU staffing, including physician staffing, derives from quasi-experimental or observational studies.41 Additional interventions occurred concurrently with the reorganization of respiratory care services. These included the implementation of a 24-hour in-house intensivist and the addition of a clinical pharmacist to the care team.15 It is difficult to isolate their relative contribution to the overall change in resource utilization, though we believe that it was the combination of these that maximized the benefit of the RT intervention.
While a clustered randomized trial examining the direct effects of RT staffing in the ICU would be ideal, this may not be feasible. While protocol-directed ventilator liberation was present prior to the intervention, computerized order reconciliation for respiratory care services was implemented after our intervention, which may have also increased adherence to respiratory care protocols. The notably low number of SBTs pre-intervention suggests either difficulties adhering to daily ventilation trial protocols or a lack of documentation, both of which would have been improved by increased RT staffing and improved training. The motivation of the RTs may have increased post-intervention, as locum tenens employees were eliminated and the remaining RTs were therefore considered long-term staff. This is an inherent component of the reorganization of the ICU post-intervention.
While SBTs are considered standard of care with regard to ventilator liberation, and lower respiratory tract culture sampling is best practice in the management of VAP, the benefit of some procedures may be less clear. For example, multiple studies of chest physiotherapy suggest little benefit when it is used in isolation,42–44 while the data on incentive spirometry are conflicting.45–47 Inhaled nitric oxide is of high cost and has not been shown to provide improvements in outcome.48 Thus, in appraising these results, a distinction may be required in parsing the relative benefits of each change in respiratory care delivery, and the reader should consider that analysis of the appropriateness of each procedure in a patient-specific context is beyond the scope of this study.
Expanding the number of RTs available in an ICU requires additional resources for salary support. Additionally, improved orientation requires substantial resources for teaching and training. This includes the extended training interval during which the hospital supports trainees until their completion of classroom instruction and training. We did find that these costs were offset by greater stability in staffing and greater productivity. Review of budget data for full medical center respiratory care services revealed the cost of training new RTs was $1,000 per week per RT. Fiscal year 2005 (July 1 through June 30), pre-intervention, was compared to 2008, post-intervention. In 2005 the budget included 36.47 full-time equivalents (FTEs) of locum tenens RTs, 14.1 FTEs of certified RTs, and 15.8 FTEs of registered RTs. There were 3 FTEs of coordinators, and a 0.9 FTE supervisor. The total 2005 budget was $5,485,111 for all staffing and training. In 2008 the budget included 0.83 FTEs of locum tenens RTs, 7.10 FTEs of certified RTs, 73.75 FTEs of registered RTs, 2.70 FTEs of supervisors, 4.88 FTEs of coordinators, and a 1.0 FTE educator. The total 2008 budget for all staffing and training was $5,569,179. Relative value units billed increased from 10,087,739 in 2005 to 11,636,577 in 2008. In summary, the relative value units per dollar generated rose from 1.84 pre-intervention to 2.09 post-intervention.
It is our belief that these added costs are justified by increased adherence to evidence-based practice and are cost-effective due to greater efficiency. However, a comprehensive analysis of the cost-effectiveness of this intervention is outside the scope of this study. As a large, tertiary care center, our results may not apply to other settings.
Conclusions
A multi-component intervention, including an increase in RT/patient ratio, improved RT orientation, and the establishment of a core staffing model was associated with increased respiratory resource utilization and evidence-based respiratory care, specifically SBTs and BALs.
Acknowledgment
We are grateful to Jeff Ford MHA RRT for his abstraction and analysis of budget data.
Footnotes
- Correspondence: Ann M Parker MD, the Division of Pulmonary and Critical Care Medicine, Johns Hopkins University, 1830 E Monument St, Baltimore MD 21205. E-mail: aparke36{at}jhu.edu.
Dr Parker presented a version of this paper at the Chest 2010 Conference, held October 30 through November 4, 2010, in Vancouver, British Columbia, Canada,
Editor in Chief Dean Hess was blinded to the peer review of this paper, which was managed by Deputy Editor Richard Branson. Dr Netzer was supported by a National Institutes of Health Clinical Research Career Development Award. Dr Harris was supported by a National Institutes of Health Midcareer Investigator Grant.
- Copyright © 2013 by Daedalus Enterprises