Abstract
BACKGROUND: Home noninvasive ventilation (NIV) is increasingly used in amyotrophic lateral sclerosis (ALS) to improve symptoms and survival. Our primary objective was to compare intelligent volume-assured pressure support (iVAPS) versus spontaneous/timed (S/T) modes regarding time to first change in ventilator parameters and the number of interventions over 6 months in subjects with ALS in a respiratory therapist (RT)-led program.
METHODS: In this study, 30 subjects with ALS meeting criteria for NIV initiation were randomized to iVAPS or S/T. NIV was initiated using standardized protocols targeting optimal tidal volume and comfort in a daytime session. Download data were recorded at 1 week and 1 and 6 months. Any changes in ventilator parameters were recorded.
RESULTS: Of the 30 subjects, 56.7% had bulbar onset ALS, 8 died, and 11 in each group completed the study. Median time to first parameter change was 33.5 (interquartile range [IQR] 7.7–96.0) d versus 41.0 (IQR 12.5–216.5) d for iVAPS versus S/T groups, respectively, (P = .48). The average number of RT interventions was similar between groups (1.1 ± 1.1 vs 0.9 ± 0.9 at 1 month, P = .72; 2.4 ± 2.1 vs 2.4 ± 2.3 at 6 months, P = .95, for iVAPS vs S/T, respectively). Adherence was significantly lower with iVAPS than S/T at 1 week but not at 1 or 6 months. Download parameters were similar between groups at 1 week and 6 months except for higher residual apnea-hypopnea index (AHI) and less spontaneously triggered breaths with iVAPS at 6 months.
CONCLUSIONS: The time to first change of parameters and the number of interventions at 6 months from NIV initiation were similar for the iVAPS and S/T modes in subjects with ALS. With iVAPS, adherence was lower transiently at NIV initiation, and the residual AHI was higher at 6 months. Alveolar ventilation-targeted NIV may require a longer adaptation period and result in greater upper-airway instability predominantly in patients with bulbar ALS.
- noninvasive ventilation (NIV)
- intelligent volume-assured pressure support (iVAPS)
- bi-level spontaneous timed ventilation
- amyotrophic lateral sclerosis (ALS)
- respiratory therapist interventions
- apnea-hypopnea index
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder that is incurable and leads to progressive respiratory muscle weakness.1 A recent systematic review and meta-analysis demonstrated that the overall worldwide ALS prevalence and incidence were 4.42 (95% CI 3.92–4.96) per 100,000 population and 1.59 (95% CI 1.39–1.81) per 100,000 person-y, respectively.2 The most common causes of death in patients with ALS are chronic hypercapnic respiratory failure, airway obstruction by secretions due to ineffective cough, and aspiration pneumonia.3,4 Home noninvasive ventilation (NIV) is increasingly used in patients with ALS for improving respiratory function, health-related quality of life, and survival.5-18 The randomized controlled trial (RCT) by Bourke and colleagues showed that NIV improves survival and quality of life in subjects with ALS with orthopnea, maximum inspiratory pressure < 60% of predicted or symptomatic hypercapnia, but without severe bulbar dysfunction.10 No new RCT has been published in subjects with ALS addressing survival and quality of life with NIV.19 However, the recent well-designed retrospective study by Ackrivo and colleagues demonstrated that NIV was associated with a 26% reduction in mortality, particularly in the limb-onset ALS subtype and with NIV use ≥ 4 h/d.18 Subjects with limb-onset ALS were more likely to tolerate NIV compared to subjects with bulbar-onset ALS (odds ratio 6.25 [95% CI 1.09–33.33]).6,20 Recently, a prospective study reported that NIV conferred a significant survival advantage even in those with severe bulbar impairment with median survival of 13 months compared with 3 months in those not using NIV (P < .001). However, bulbar impairment was a cause of NIV failure in 49% of cases and a major prognostic factor.21
Recently, the Canadian Thoracic Society published guidelines for long-term NIV in patients with ALS.22 They suggested earlier initiation of NIV in ALS compared with previous guidelines based on studies suggesting that earlier initiation of NIV in patients with ALS results in improved rate of decline in respiratory function, survival, and quality of life.10,23,24 Data remain limited regarding optimal ventilation modes and parameters, and no recommendation could be made regarding volume-assured pressure support (VAPS) modes of NIV. The intelligent VAPS (iVAPS) is an adaptive ventilation mode that uses an algorithm to adjust pressure support to target a specified alveolar ventilation.25 It has a learning mode that uses the baseline awake ventilation to determine the settings for ventilation.
Several RCTs showed similar efficacy of iVAPS compared to standard pressure support ventilation (PSV) on improving gas exchange, pulmonary function, sleep disturbances, and adherence in individuals with obstructive or restrictive lung disease with chronic hypoventilation.26-32 Interestingly, one RCT showed that the adherence to iVAPS over 1 month was better than standard PSV in a mixed population (5.4 vs 4.2 h/night for iVAPS vs PSV; P < .01). iVAPS delivered a lower median pressure support compared with standard PSV.32 The principle of iVAPS is theoretically well suited for ALS due to its adaptive response that obviates the need for overnight NIV titration and its ability to react to progressively deteriorating spontaneous respiratory function over time. Progression of ventilatory failure may result in recurrent periods of inadequate ventilation if parameter adjustments cannot be made, with reduced quality of life and more stress related to respiratory or sleep-related symptoms.33-35 Hence, a device that can be titrated in a daytime trial and would self-adjust automatically over time as respiratory muscle weakness progresses could be beneficial for patient-related outcomes and could be more cost effective. On the other hand, patients with ALS may have difficulty tolerating NIV and may require an adaptation period with progressive increase in NIV support.32 A recent retrospective study comparing iVAPS to standard PSV in subjects with ALS showed that iVAPS provided more reliable target tidal volume (VT) than PSV and was associated with decreased rapid shallow breathing index (f/VT) as an indicator of work of breathing.36 However, whether iVAPS results in fewer respiratory therapist interventions related to NIV than standard pressure modes has not been studied. Therefore, the primary aim of this study was to compare iVAPS and spontaneous/timed (S/T) modes in subjects with ALS with respect to time to first change in ventilator parameters and the number of interventions over 6 months, following daytime NIV initiation in the context of a respiratory therapist (RT)–led home ventilation program.
QUICK LOOK
Current Knowledge
Home noninvasive ventilation (NIV) is increasingly used in amyotrophic lateral sclerosis (ALS) and improves symptoms and survival. The intelligent volume-assured pressure support (iVAPS) is an adaptive NIV mode that targets alveolar ventilation and might be helpful for ALS in the context of progressively deteriorating respiratory function over time. Previous studies demonstrated that the iVAPS might impact comfort and adherence to treatment in patients with neuromuscular disease with hypoventilation.
Contributes to our knowledge
In this pilot trial, iVAPS did not require fewer interventions or parameter adjustments than the spontaneous/timed mode over 6 months from NIV initiation in a group of subjects with ALS, including a large proportion of individuals with bulbar-onset ALS. Adherence to iVAPS was transiently lower, suggesting that this mode may require a longer adaptation period in patients with ALS. Adherence and subject symptoms were similar with both NIV modes.
Methods
We performed a pilot RCT with recruitment from February 2014–September 2018 of patients referred for home NIV to the National Program for Home Ventilatory Assistance (NPHVA or Program National d’Assistance Ventilatoire á Domicile, PNAVD) of the McGill University Health Centre (MUHC). Consecutive patients with ALS meeting criteria for NIV initiation were approached. Criteria for NIV were the presence of hypoventilation symptoms and at least one criterion such as daytime hypercapnia (PaCO2 > 45 mm Hg), orthopnea, nocturnal hypoventilation (PCO2 during sleep increase > 10 mm Hg compared with awake PCO2 or nocturnal oxygen desaturation < 88% for a period of at least 5 consecutive min), FVC < 50% of predicted, sniff nasal inspiratory pressure (SNIP) < −40 cm H2O, or PImax < −40 cm H2O. Patients were excluded if they had other major neurological disorders or active cancer, were referred from hospital and were already on a mechanical ventilator, or could not provide informed consent. This study has been registered on ClinicalTrials.gov (NCT01746381). The MUHC Research Ethics Board approved the study protocol. All subjects gave written informed consent.
Subjects with ALS were randomized 1:1 to iVAPS or S/T mode, both with a ResMed Stellar 150 ventilator (ResMed, San Diego, California). Randomization was performed using numbered sealed opaque envelopes based on a random sequence generator. NIV was initiated by an experienced RT using standardized protocols targeting optimal VT and comfort in a daytime session in an out-patient day hospital setting lasting approximately 3 h. Subjects were encouraged to sleep while titration was ongoing if possible. For subjects randomized to S/T mode, expiratory positive airway pressure (EPAP) was set at 5 cm H2O, pressure support was adjusted to achieve optimal VT (8 mL/ideal body weight) if tolerated, and the backup frequency was set just below the subject’s spontaneous breathing frequency, aiming to maximize the subject’s comfort. For those randomized to iVAPS, the learning mode was used to determine the target alveolar ventilation as per the manufacturers protocol.25 The RT selected the 5-min window where the subject’s ventilation and breathing frequency looked stable to set up target alveolar ventilation and backup frequency (as per the “intelligent backup rate” algorithm). Parameters were then tested and adjusted if needed by increasing the minimum level of pressure support to target VT of 8 mL/ideal body weight and for the subject’s comfort. The RT also adjusted inspiratory time, rise time, fall time, trigger sensitivity, and cycle sensitivity for each subject’s comfort in both iVAPS and S/T groups. If a subject fell asleep during the trial and obstructive events were noted, EPAP was raised manually for both S/T and iVAPS modes. For all subjects, the RT recorded vital signs, oxygen saturation (SpO2), and capillary blood gases before the trial of ventilation. All subjects underwent spirometry37 and a SNIP test,38-39 including SNIPopen and SNIPclosed40 at baseline.
The RT visited all subjects at home at 1 week and at 1 and 6 months after NIV initiation. Download parameters such as adherance data (hours of daily usage), air leak, ventilator pressures used, minute ventilation, and respiratory events were recorded. Adjustments to ventilator settings were made if deemed necessary to optimize ventilation, minimize the apnea-hypopnea index (AHI), and maximize comfort and subject symptoms for both iVAPS and S/T groups. Adjustments could be initiated by the RT with pulmonologist approval or by the supervising pulmonologist based on downloaded data. Any changes were recorded. In addition, daytime SpO2 and end-tidal CO2 (EtCO2) were measured at baseline, 1 month, and 6 months. Overnight oximetry also was assessed at 1 month and 6 months using the Rad-8, Masimo SET (Masimo, Irvine, California). Health-related quality of life was assessed at baseline and 3 months using the Severe Respiratory Insufficiency (SRI) questionnaires (scores range from 0–100, with higher scores indicating a better quality of life).41 Participants’ sleep-related symptoms and ventilator satisfaction were assessed at baseline and 6 months using our own questionnaire (see related supplementary materials at http://www.rcjournal.com).
Statistical Analysis
The sample size for this pilot study was calculated based on the expected number of ventilator changes over 6 months. We assumed a difference of 2 interventions between the study arms and an SD of 1.5. We would then need 10 subjects per group to detect this difference with alpha 0.05 and power 80%. To account for withdrawal from the study or premature death, the total planned sample size was 30 subjects. Descriptive statistics were provided for each group. Continuous variables were reported as mean and SD. Binary and categorical variables were summarized using frequency counts and percentages. Comparisons of continuous variables were performed using the t test and of categorical variables with the chi square test. Pearson correlation was used to determine variables associated with adherence to NIV. Time to first ventilator change was analyzed using survival analysis and Kaplan-Meyer plots with a log-rank test. A P value of < .05 was considered statistically significant. All analyses were carried out using SPSS software, version 27 (IBM, Armonk, NY).42
Results
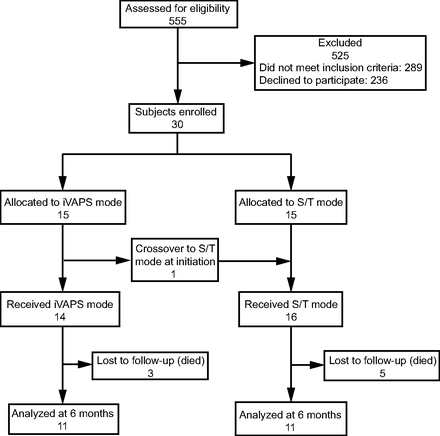
Five hundred fifty-five patients with ALS were referred to the NPHVA between February 2014–September 2018. Of these, 289 patients were ineligible on the basis of not meeting criteria for NIV initiation, and 236 patients declined to participate in the study. Thirty subjects were randomized to iVAPS or S/T mode. One subject crossed over from iVAPS to S/T due to intolerance at NIV initiation. At 6 months, a total of 3 and 5 subjects died in the iVAPS and S/T groups, respectively. There remained 11 subjects in each group who completed follow-up at 6 months (Fig. 1). Participants’ mean age was 62.8 ± 9.5 y, and 53.3% were male (Table 1). Most subjects were non-obese. Over half (56.7%) of the subjects had bulbar onset ALS. A minority (13.3%) of subjects had feeding tubes. Most subjects had daytime normocapnia (PaCO2 = 42.5 ± 3.8 mm Hg) and normal oxygen saturation (SpO2 = 95.9 ± 2.4%) at NIV initiation. On average, the subjects had moderately severe restrictive lung disease based on FVC (55.4 ± 17.8% of predicted at enrollment). At baseline, no significant differences in demographics, health-related quality of life (SRI), gas exchange, and pulmonary function were observed between iVAPS and S/T groups except for higher proportions of wheelchair users and pregabalin users in the S/T group and higher daytime PaCO2 in the iVAPS group (Table 1).
Flow chart. iVAPS = intelligent volume-assured pressure support; S/T = spontaneous/timed.
Baseline Demographics, Blood Gas, and Pulmonary Function Parameters for Subjects With Amyotrophic Lateral Sclerosis Using Intelligent Volume-Assured Pressure Support Versus Spontaneous/Timed Modes
Table 2 shows the ventilator settings in iVAPS and S/T groups at 1 week, 1 month, and 6 months. At 1 week, the backup frequency was significantly higher in the iVAPS group at 15.4 versus 11.4 breaths/min for iVAPS versus S/T groups, respectively, (P = .004). Adherence to NIV was significantly lower with iVAPS than S/T at 1 week (Table 3). At 1 week, 13.2% of participants in the iVAPS group was using NIV ≥ 4 h/night compared with 45.4% using S/T (P = .033). The average duration of NIV use at 1 week was 2.2 and 4.6 h/night for iVAPS versus S/T groups, respectively, (P = .034). However, there were no significant differences in NIV adherence between groups at 1 and 6 months. (Table 3). Download parameters were similar between groups at 1 week, 1 month, and 6 months (Table 3) except for significantly higher residual AHI and hypopnea index with iVAPS at all time points and less spontaneously triggered breaths with iVAPS at 6 months, possibly related to differences in trigger sensitivity. To assess if the AHI is related to variability in ventilation, we calculated Pearson correlation coefficients between the residual AHI and the difference between 95th percentile and median inspiratory positive airway pressure (Δ IPAP) for the iVAPS group. Then we found no significant relationship between the residual AHI and the Δ IPAP for the iVAPS group at any time point: r = 0.494, P = .09 at 1 week; r = 0.176, P = .57 at 1 month; and r = 0.089, P = .81 at 6 months (Figure S1, see related supplementary materials at http://www.rcjournal.com). However, we found significant correlations between the residual AHI and the difference between 95th percentile and median VT (Δ VT) for the iVAPS group at 1 and 6 months (r = 0.480, P = .08 at 1 week; r = 0.683, P = .007 at 1 month; and r = 0.706, P = .01 at 6 months) (Figure S1). In exploratory analyses, we divided subjects into bulbar and non–bulbar onset ALS. We observed a significantly higher residual AHI with iVAPS only at 1 month in subjects with bulbar-onset ALS (Tables S1 and S2, see related supplementary materials at http://www.rcjournal.com), though nonsignificant differences occurred in both groups at other time points. We used the rapid shallow breathing index (f/VT) to evaluate the work of breathing in subjects on NIV. We found no significant difference in f/VT during NIV use between groups over 6 months (Table 3). In this cohort, mask leak did not differ significantly between iVAPS and S/T (Table 3). We also found no significant relationships between average hours of NIV use and demographics, gas exchange parameters, download parameters, mask changes, or lung function parameters except for an inverse relationship with SNIPopen (r = −0.55, P = .02) (Table 4).
Noninvasive Ventilation Settings and Type of Mask at 1 Week, 1 Month, and 6 Months
Download Parameters of Noninvasive Ventilation at 1 Week, 1 Month, and 6 Months
Association Between Noninvasive Ventilation Adherence (Daily Use, All Days) and Other Variables at 6 Months
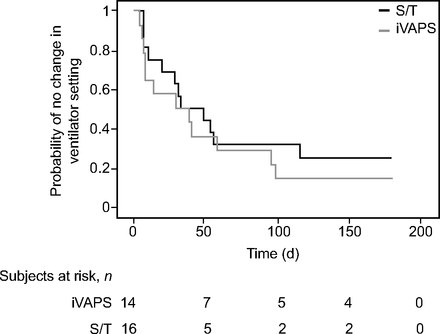
As shown in Figure 2, the median time to first ventilator parameters change was 33.5 (IQR 7.7–96.0) d versus 41.0 (IQR 12.5–216.5) d for iVAPS versus S/T groups, respectively, (P = .48 by log-rank test). The average number of RT interventions was similar between iVAPS and S/T groups at 1 month and 6 months (1.1 ± 1.1 vs 0.9 ± 0.9 at 1 month, P = .72; 2.4 ± 2.1 vs 2.4 ± 2.3 at 6 months, P = .95, for iVAPS vs S/T, respectively). The characteristics of interventions were similar in both groups except for more increases in trigger sensitivity with S/T (Table 5). No significant difference in interventions to optimize mask fit between iVAPS and S/T groups was observed. At 6 months, 3 and 5 subjects died in the iVAPS and S/T groups, respectively, (Chi square, P = .54), all from respiratory failure.
Kaplan-Meier curves for time to first change in ventilator settings according to ventilator mode. S/T = spontaneous/timed; iVAPS = intelligent volume-assured pressure support.
Characteristics of Respiratory Interventions Over 6-Month Follow-Up
Most subjects had normal daytime oxygen saturation and EtCO2 at baseline, 1 month, and 6 months. We found that daytime oxygen saturation and EtCO2 in iVAPS were similar to the S/T group from NIV initiation to 1 month and 6 months follow-up (Table S3, see related supplementary materials at http://www.rcjournal.com). Six participants underwent overnight oximetry at 1 month and 6 months in iVAPS and S/T groups. We found no difference in the mean or nadir SpO2 during sleep, 4% oxygen desaturation index, and percentage time with oxygen saturation < 90% from 1 month to 6 months between iVAPS and S/T groups (Table S3). We also observed no significant difference in participant’s sleep-related symptoms between iVAPS and S/T groups from NIV initiation to 6 months follow-up (Table S4, see related supplementary materials at http://www.rcjournal.com). According to the SRI questionnaires, there was no significant difference in health-related quality of life between iVAPS and S/T group in change from baseline to 3 months except that the respiratory-complaint component deteriorated significantly in the S/T group but not in the iVAPS group (Table S5, see related supplementary materials at http://www.rcjournal.com). In addition, no significant difference in participant-reported ventilator comfort and satisfaction was observed between iVAPS and S/T groups at 1 and 6 months (Table S6, see related supplementary materials at http://www.rcjournal.com).
Discussion
In this study, we found that time to first ventilator parameter change and number and type of RT interventions over 6 months were similar in subjects with ALS using iVAPS and S/T modes of NIV. This study demonstrated that the iVAPS mode did not require fewer interventions than the S/T mode over 6 months from NIV initiation. The iVAPS mode was as effective as standard S/T pressure ventilation when initiated by a highly skilled RT in a home ventilation program with respect to physiologic and patient-related outcomes. We also found ventilator download parameters, daytime gas exchange, ventilator comfort, and respiratory symptoms to be similar between groups except for higher residual AHI on iVAPS. These findings support the previous studies comparing VAPS with standard PSV in subjects with neuromuscular disease.28,43
Interestingly, we found that adherence was significantly lower with iVAPS than S/T at 1 week but not at 1 or 6 months. Jaye and colleagues had found no significant difference in adherence between auto-titrating and standard PSV in neuromuscular disease.28 On the other hand, this finding is contrary to the previous RCT by Kelly and colleagues, which suggested that the adherence to iVAPS over 1 month was better than standard PSV and lower median pressure support with iVAPS in a mixed population.32 Although we demonstrated that alveolar ventilation-targeted NIV may require a longer adaptation period, there was no significant difference in adherence or survival between iVAPS and S/T at 6 months among our subjects with ALS. We hypothesize that a longer adaptation period may be needed in subjects with ALS with bulbar impairment to the iVAPS mode that is inherently less stable than the S/T mode. Patients with bulbar symptoms may be more prone to reflex laryngospasm,44-46 glottic closure, or discoordination with more variable pressure, but we have not assessed this systematically. An important element associated with adherence to NIV is adequate mask fit. Whereas there were more mask changes in the S/T group in our study, the difference was not statistically significant, and similar proportions of subjects used facial versus nasal masks in each group. We found no significant relationship between NIV adherence and mask changes (Table 4). Irrespective of NIV mode, adherence was related to lower SNIP (Table 4), which suggests that greater diaphragm weakness is the main factor driving patients with ALS to use NIV.
Nicholson and colleagues36 found that adequate VT were more reliably attained for iVAPS compared with PSV in subjects with ALS. However, we found no significant difference in median VT between iVAPS and S/T modes over 6 months. They also reported a significantly lower rapid shallow breathing index with iVAPS compared to PSV, whereas we found no difference. This may be due to differences in the study populations (more bulbar symptoms and higher FVC in our study population) and in the effectiveness of the parameters set for the S/T mode. In our study, participants in the S/T group achieved higher VT and a lower rapid shallow breathing index than the S/T group in the Nicholson study.
The learning mode in iVAPS uses the patient’s respiratory pattern to set the ventilation parameters. In patients with ALS, it is prone to “learning” the patient’s dysfunctional rapid shallow breathing pattern that results from respiratory muscle weakness. It would then apply inadequate NIV settings with underestimated pressure support. Both the Nicholson and our study protocols have ensured the minimum pressure support setting is manually corrected if needed to provide sufficient VT.
Previous studies reported that subjects with limb-onset ALS were more likely to tolerate NIV compared with subjects with bulbar-onset ALS.10,11,20 We found no significant relationships between average hours of NIV use and type of onset of ALS. That is, adherence was no different for bulbar versus non–bulbar subjects. Several studies showed bulbar-onset ALS had a greater risk for upper-airway obstruction during sleep.34,47,48 We found significantly higher residual AHI in subjects using iVAPS, primarily but not exclusively in subjects with bulbar-onset ALS. We hypothesize that the iVAPS mode, with fluctuating levels of pressure support, might result in greater upper-airway instability predominantly in individuals with bulbar ALS. Moreover, the iVAPS mode may have resulted in transient intermittent overventilation and hypocapnia that resulted in central or obstructive hypopneas, despite relatively low levels of pressure support. This hypothesis is supported by our finding of significant correlations between the residual AHI and Δ VT for the iVAPS group. Larger studies, ideally with polysomnographic confirmation, will be needed to confirm this hypothesis.
The strength of our study is that we studied subjects with ALS for a period of 6 months, though with several deaths during the study period. We assessed a wide range of outcomes, and although this has led to multiple comparisons, results should be seen as exploratory for this pilot study. There were some limitations to our study. First, participants and RTs were not blinded to the intervention, which may have introduced bias. However, this would have been expected to result in fewer interventions or longer time to interventions in the iVAPS mode, which was not the case. Second, our findings were based on a small sample of subjects because of its pilot nature. Third, we did not evaluate quality of life in our subjects at 6 months because most subjects refused to do the SRI questionnaires at 6 months and some subjects died before 6 months of follow-up. Furthermore, unexpectedly, over half of the subjects had bulbar-onset ALS. These results might, therefore, not be representative of a more typical group of patients with ALS with a larger proportion of non–bulbar-onset initiating NIV. There were other unbalanced baseline characteristics between groups that may have impacted results such as medication differences and statistically but not clinically important PaCO2 differences. Additionally, we did not evaluate baseline polysomnography to evaluate the prevalence of sleep-disordered breathing before NIV initiation, nor were sleep-disordered breathing and gas exchange assessed in all patients on NIV at follow-up. A future larger randomized control study comparing iVAPS with standard S/T mode with overnight gas exchange monitoring at baseline and follow-up would be useful to better understand differences between the 2 modes in both subjects with bulbar and non–bulbar-onset ALS. Finally, this study was conducted in the context of a large, well-established home ventilation program staffed by highly trained and experienced RTs accustomed to working with the ALS population. Conceivably, the iVAPS mode could provide advantages for adaptation to therapy over S/T in other clinical contexts, although further research would be required to specifically address this question.
Conclusions
The iVAPS mode did not require fewer interventions than the S/T mode over 6 months from NIV initiation in subjects with ALS. With iVAPS, adherence was transiently lower at NIV initiation, and the residual AHI was higher at 6 months. Alveolar ventilation-targeted NIV may require a longer adaptation period and result in greater upper-airway instability in patients with ALS.
ACKNOWLEDGMENTS
We would like to thank the whole team of the Quebec NPHVA, MUHC, Montreal, Quebec, Canada. We would like to acknowledge Dr Lancelot Pinto for his work on developing the questionnaires for the study.
Footnotes
- Correspondence: Pattaraporn Panyarath MD, McGill University Health Centre, Respiratory Division/Sleep laboratory, 1001 Decarie Boulevard, Montreal, Quebec, Canada, H4A 3J1. E-mail: pattaraporn.panyarath{at}mail.mcgill.ca
The authors have disclosed a relationship with ResMed.
This study was funded by an unrestricted grant and in-kind (ventilator device) support from ResMed. ResMed had no input in the design, conduct, analysis, or publication of this study.
This study was performed at McGill University Health Centre and Quebec National Program for Home Ventilatory Assistance, Montreal, Quebec, Canada.
Supplementary material related to this paper is available at http://www.rcjournal.com.
- Copyright © 2022 by Daedalus Enterprises