Abstract
OBJECTIVE: To evaluate optimal humidifier water temperature when using a helmet for noninvasive ventilation.
METHODS: Twenty-eight healthy individuals underwent 8 cm H2O CPAP ventilation with FIO2 of 0.21 and 0.5. Each was sequentially tested in the following order: using the helmet without humidification at ambient temperature; with humidification with unheated chamber water; and with humidification with the chamber water at 31°C, 34°C, and 37°C. At each setting, after a 20 min stabilization period, measurements were taken. Comfort level at each setting was evaluated using a visual analog scale rated zero (least comfortable) to 10 (most comfortable).
RESULTS: Temperature and relative and absolute humidity inside the helmet increased; however, the comfort scores significantly decreased as the humidification chamber water temperature increased. Regardless of the FIO2, statistically significantly highest comfort scores were obtained when humidification water, with and without active humidification, was at ambient temperature. Unacceptable absolute humidity was obtained only without humidification at room temperature when FIO2 was 0.5.
CONCLUSIONS: With the clinical use of a helmet, for patient comfort and mucosal humidification during CPAP, the most desirable conditions are likely to be obtained by humidifying without heating, that is by leaving the water in the humidifier chamber at room temperature.
Introduction
Noninvasive ventilation (NIV) for the treatment of acute and chronic respiratory failure has become increasingly important during the last decade.1 Although NIV has been widely delivered by means of nasal or oronasal masks, air leakage, patient discomfort, skin lesions, and other problems may lead to the failure of NIV.1,2 One study3 has reported that the choice of delivery method improves the performance of NIV more than the mode of ventilation.
Recently, ventilation helmets have been developed to improve NIV tolerance and problems with other methods of delivery.1 It has been reported that the use of helmets with acute respiratory failure patients is better tolerated, improves PaO2, and entails fewer complications than when conventional oronasal masks are used,4 and may sometimes be preferred despite increased noise exposure, carbon dioxide rebreathing, patient-ventilator asynchrony, and increased work of breathing.1 We found that some infants sweated, however, and were unable to tolerate helmet moisture for even 1 hour, forcing us to abandon our helmet-mode NIV trials. We found 2 reports investigating humidification during NIV: Chiumello and colleagues5 only tested flow rate at a fixed temperature setting (37°C), and Lellouche and colleagues6 tested with different temperature settings, but using a nasal mask rather than a helmet. Although temperature and humidification inside a helmet are affected by various factors, such as type of ventilator, circuit length, and room temperature, during NIV delivered by helmet to healthy subjects we investigated what conditions of the inspired gas, including temperature and absolute and relative humidity, could be considerate adequate, as well as other factors such as FIO2 that might influence the absolute humidity of inspired gases. We conjectured that comfort would be greatest with humidification when inspiratory gas temperature was close to ambient.
QUICK LOOK
Current knowledge
During traditional noninvasive ventilation (NIV) with a nasal or oronasal mask, humidification of the inspired gas improves patient comfort and NIV tolerance, but the optimum temperature and humidity are unknown. The NIV helmet may reduce patient discomfort, but heating and humidification during helmet NIV are controversial.
What this paper contributes to our knowledge
In healthy volunteers, NIV helmet had better comfort with unheated humidification. Increasing the temperature of the inspired gas was directly related to increasing discomfort.
Methods
For this study, approved by the institutional review board of Osaka University Hospital, we recruited 28 healthy adult subjects (19 male, 9 female). In accordance with Japanese regulations, written informed consent was obtained from each subject.
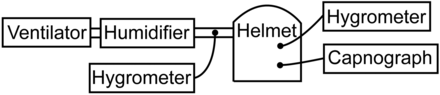
We used transparent, latex-free helmets (CaStar, StarMed, Modena, Italy) that basically comprise a transparent rigid polyvinyl chloride tube sealed at the top and connected at the bottom by a metal ring to a soft polyvinyl chloride collar. Exhalation was via an expiratory port in a flat cap (diameter 4 mm, SPV12F, StarMed, Modena, Italy). The absence of excessive air leakage was confirmed by viewing the graphical monitor on the ventilator. The subjects were tested in the sitting position. CPAP mode was administered via standard plastic disposable circuits, using a BiPAP Vision ventilator (Respironics, Murrysville, Pennsylvania) and a heated humidifier (MR 730, Fisher & Paykel, Auckland, New Zealand). Water in the humidifier reservoir was heated until the gas at the end of the inspiratory line reached the preset temperature.5 FIO2 was set at 0.21 and 0.5, and CPAP at 8 cm H2O.
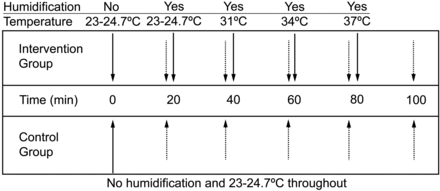
In the protocol, each subject was first evaluated after receiving, without water in the humidifier container, ventilation at ambient temperature via the helmet for 20 min, at which time the visual analog scale comfort score was elicited and other readings taken. Following this, the humidifier chamber was filled with unheated water and similar evaluations were sequentially made after each 20 min period of controlled conditions with the chamber water unheated, and heated to 31°C, 34°C, and 37°C (Fig. 1). Based on results of a preliminary study (data not shown), we were confident that 20 min was adequate for stabilization. The settings were not randomized, because moisture might remain in the circuit and helmet if a higher temperature setting preceded a lower temperature setting. For control, 4 subjects underwent helmet ventilation at ambient temperature without humidification for 100 min. During the protocol, the subjects were not informed of the humidifier settings. The following measurements were recorded at the end of each 20 min period: breathing frequency; end-tidal CO2 (PETCO2); and, both inside the helmet and at the inspiratory connector of the helmet, relative humidity, absolute humidity, and temperature. Breathing frequency and PETCO2 were measured using an OxiMaxN-85 (Nellcor/Covidien, Boulder, Colorado). Temperature and humidity readings (Fig. 2) were taken using a MoiScope (Senko Medical Instruments, Tokyo, Japan) and additionally, when FIO2 was 0.5, using a HygroPalm (Rotronic, Bassersdorf, Switzerland). The American National Standards Institute suggested, although not directly for NIV, that 10 mg H2O/L of absolute humidity is the lowest acceptable level needed to minimize mucosal damage in the upper airways.5 Subjective comfort was evaluated using a visual analog scale rated from zero (least comfortable) to 10 (most comfortable).
Experimental protocol for intervention and control groups. The room temperature range was 23–24.7°C.
Experimental setup.
Values are expressed as mean ± standard deviation for parametric, or median (interquartile range) for nonparametric distribution. Data were analyzed with repeated measures analysis of variance with StatView 5 statistics software (SAS Institute, Cary, North Carolina) and the Tukey-Kramer method using JMP 8 (SAS Institute, Cary, North Carolina). P values of < .05 were considered significant.
Results
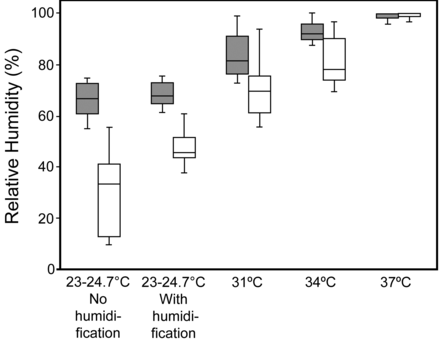
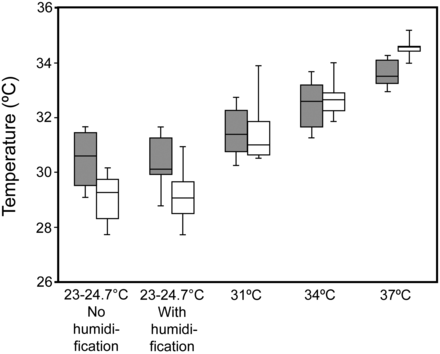
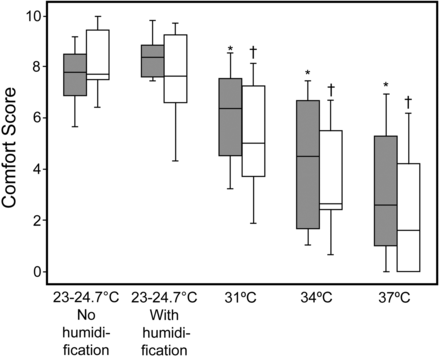
During ventilation delivered by helmet, the measured room temperature ranged between 23°C and 24.7°C. During CPAP, the mean breathing frequency was 14.3–17 breaths/min (Table 1). The MoiScope readings of temperature and relative humidity inside the helmet were used because they were not statistically significantly different from the HygroPalm readings when FIO2 was 0.5: temperature 31.4 ± 2.3°C vs 31.1 ± 2.4°C, relative humidity 68.8 ± 46.8% vs 67.3 ± 46.4%. In humidified conditions, regardless of FIO2, when the temperature of the water in the humidification chamber increased, along with temperature, relative and absolute humidity statistically significantly increased inside and outside the helmet, and the subjects reported greater discomfort (Figs. 3, 4, and 5, and see Tables 1 and 2). The comfort scores were significantly highest at ambient temperature, with and without humidification, both at FIO2 0.21 and at FIO2 0.5 (Fig. 6 and see Table 1). The minimum absolute humidity was 9.6 (3.4–12.3) mg H2O/L, found in 7 subjects out of 12 (58%), which was the only unacceptable level in this study; it was recorded when applying FIO2 of 0.5 at ambient temperature without humidification. We found no statistically significant changes in PETCO2 during the different conditions of the protocol (Tables 1 and 3). For the control group we did not find any statistically significant changes in data gathered at the 20 min sampling points (see Table 3).
Respiratory Variables, Comfort Score, Temperature, and Humidity
Relative humidity inside the helmet. Shaded bars: FIO2 0.21. White bars: FIO2 0.5. The solid horizontal lines represent the medians. The tops and bottoms of the bars represent the 25th and 75th percentiles. The whisker bars represent the 5th–95th percentiles. The room temperature range was 23–24.7°C. At 31°C, 34°C, and 37°C the circuit was humidified.
Absolute humidity inside the helmet. Bars and humidification as in Figure 3.
Temperature inside the helmet. Bars and humidification as in Figure 3.
Temperature and Humidity Outside Helmet
Comfort scores. Bars and humidification as in Figure 3. * Significant difference compared with room temperature humidified and not humidified at FIO2 of 0.21. † Significant difference compared with room temperature humidified and not humidified at FIO2 of 0.50.
Control Group Respiratory Variables, Comfort Score, and Temperature and Humidity Inside the Helmet*
Discussion
This study revealed that discomfort increases as humidifier chamber water temperature rises, which results in higher temperature and increased relative and absolute humidity inside the helmet. At either of the tested FIO2 settings (0.21 and 0.5), whether humidification was applied or not, subjects were most comfortable at ambient temperature. To our knowledge, this is the first report to clarify the importance of temperature setting during helmet NIV.
NIV has usually been administered via oronasal mask to patients with acute respiratory failure. Delivery by oronasal mask, however, may cause discomfort and skin lesions. Oronasal masks are also susceptible to leakage5,7 that may lead to ineffective ventilation. Helmet delivery, regardless of the anatomical structure of the face and neck, presents less risk of skin lesion and air leakage.8 Another benefit is easier verbal and facial communication, which enhances patient comfort and improves the feasibility of longer periods of NIV.5 Against this must be weighed the problems of increased noise, carbon dioxide rebreathing, and patient-ventilator asynchrony.1
A recent review of NIV9 concluded that humidification is usually unnecessary for short periods of CPAP ventilation. By contrast, the International Consensus Conference on NIV in Intensive Care7 advised that inadequate humidification may cause patient distress, especially if piped or cylinder gas is used. Along with a number of other reports,5,10–12 we found that heated air increases both absolute and relative humidity during NIV, because the humidifier heating delivers enough energy to the water in the humidification chamber.5 While this seems to suggest that heated humidification may be needed for conventional CPAP using an oronasal or nasal mask, few studies have reported optimal humidifier chamber water temperatures during nasal CPAP.11 Although when inspired flow is medium or low (FIO2 of 0.21),5 humidification during delivery by helmet may be less necessary than during other modes, because inspired ambient air mixes with expired humidified gas. In this study, when FIO2 of 0.5 was applied, the greater proportion of dry medical gas reduced the humidity of the inspired gas so that, at ambient temperature without humidification, we found a minimum absolute humidity of 9.6 (3.4–12.3) mg H2O/L, an unacceptable level that is likely to cause perceptible mucosal damage in the upper airways.13 Our findings suggest that for higher FIO2, which is often clinically applied for lung-injured patients during helmet NIV, humidification is necessary during delivery by helmet.
Owing both directly to higher in-helmet temperatures and, consequently, to increasing relative and absolute humidity inside the helmet, our subjects complained of feeling feverish when the humidifier heater was set at 37°C. They also disliked the way that condensation fogged the helmet at temperatures of more than 34°C. Greater discomfort was reported each time the humidifier water temperature was increased. Subjects in this study scored helmet ventilation accompanied by humidification with unheated humidification chamber water or without humidification as the most comfortable, regardless of whether the FIO2 was set 0.21 or 0.5. Sustainable long-term comfort is especially important, because longer toleration of NIV allows the potential advantages of NIV to become apparent.1 Our findings provide evidence that, during clinical delivery of inspiratory gas by helmet NIV, humidification at ambient temperature is desirable for patient comfort, as well as for preventing mucosal damage. While we did find one report suggesting that it is necessary to apply heated humidification when using a helmet for high-flow CPAP ventilation,5 only one temperature, 37°C, was tested. At lower temperature settings, significantly less discomfort was reported. In another study, during biphasic positive airway pressure ventilation, helmeted patients similarly complained of feeling hot, because the temperature inside the helmet increased.14 In another study, heated humidification was probably necessary for half the patients tested, whose nasal discomfort led to the abandonment of unheated humidification during nasal CPAP.15 Another report, while acknowledging the necessity of further study to clarify the possible benefit of heated humidification with nasal CPAP, found that the lesser humidification capacity of non-heated passover humidifiers may be sufficient to prevent airway dryness during clinical nasal CPAP ventilation.11 Although helmets and oronasal masks create different conditions around the head, comfort data from another report on delivery via oronasal mask suggest that levels above 15mg H2O/L are well tolerated with heated humidification during CPAP.6
Because it was not practical to randomize the variation of humidifier water temperature, we set up a control group to receive helmet ventilation for 100 min at ambient temperature without a humidifier. Thus, we were able to exclude the influence of time spent in the helmet. We found no significant differences in control group comfort score data during the protocol. At least for periods of up to 100 min, this indicates that duration has no major effect on comfort.
The study has some obvious limitations. For example, the protocol lasted less than 2 hours, and it would be imprudent to simply assume that these results can be extended to longer periods of helmet ventilation. Neither did we evaluate any model of ventilator that intermittently delivers medical oxygen and medical air. During ventilator CPAP, intermittent low flow dilutes expired humidity less than the continuous gas flow we tested in this study,5 and such humidification may be sufficient to humidify during a similar protocol. Nor did we evaluate other modes of ventilation, such as pressure support ventilation, where the delay of inspiratory triggering in the helmet may cause discomfort that might increase over time. The findings of Chiumello et al5 and the current study suggest that during helmet NIV inspiratory gas conditions inside the helmet, such as humidity, temperature, and flow are the main determinants of temperature and humidity; consequently, during pressure support ventilation, similar results may also be obtained. Investigation would be required, however, to see whether the increased tidal volume occurring during pressure support ventilation causes greater retention of moisture from expired air and concomitantly greater humidity inside the helmet.
Since the temperature of the humidifier water can only be set to 31°C or more, it was impractical to design the study to evaluate comfort using water at temperatures between ambient temperature and 31°C. The optimum temperature setting may be between room temperature and 31°C. Finally, our findings from healthy subjects may not be directly applicable to situations with lung-injured patients. In our study the medium inspiratory gas flow, 30–50 L/min, through the helmet did not cause ventilatory problems; however, the lower gas flow resulted in elevated PaCO2.16 Clinical studies of CPAP ventilation using helmets have shown this mode of delivery is less effective in reducing elevated PaCO2: while higher gas flow should be considered for patients who exhibit high PaCO2,1,16 it should be borne in mind that humidification problems are more likely to occur with higher flow.17
Conclusions
In summary, discomfort increased as humidifier water temperature rose. This discomfort was attributable to increased relative and absolute humidity inside the helmet. Our current findings lead us to conclude that, for patient comfort and mucosal humidification, the most desirable currently practical humidification when delivering CPAP through a helmet is likely to be with unheated humidifier chamber water. Further study is required to ascertain the best humidification settings for other ventilators, for other modes of ventilation, and for longer periods of ventilation.
Acknowledgments
We thank Mr David Eunice for his help in revising the manuscript.
Footnotes
- Correspondence: Kazuyoshi Ueta MD, Department Anesthesiology and Intensive Care Medicine, Osaka University Graduate School of Medicine, 2–15 Yamadaoka, Suita, Osaka Japan 565-0871. E-mail: kueta{at}anes.med.osaka-u.ac.jp.
The authors have disclosed no conflicts of interest.
- Copyright © 2013 by Daedalus Enterprises