Abstract
BACKGROUND: Calculation of physiologic dead space (dead space divided by tidal volume [VD/VT]) using the Enghoff modification of the Bohr equation requires measurement of the partial pressure of mean expired CO2 (PĒCO2) by exhaled gas collection and analysis, use of a metabolic analyzer, or use of a volumetric CO2 monitor. The Dräger XL ventilator is equipped with integrated volumetric CO2 monitoring and calculates minute CO2 production (V̄CO2). We calculated PĒCO2 and VD/VT from ventilator derived volumetric CO2 measurements of V̄CO2 and compared them to metabolic analyzer and volumetric CO2 monitor measurements.
METHODS: A total of 67 measurements in 36 subjects recovering from acute lung injury or ARDS were compared. Thirty-one ventilator derived measurements were compared to measurements using 3 different metabolic analyzers, and 36 ventilator derived measurements were compared to measurements from a volumetric CO2 monitor.
RESULTS: There was a strong agreement between ventilator derived measurements and metabolic analyzer or volumetric CO2 monitor measurements of PĒCO2 and VD/VT. The correlations, bias, and precision between the ventilator and metabolic analyzer measurements for PĒCO2 were r = 0.97, r2 = 0.93 (P < .001), bias −1.04 mm Hg, and precision ± 1.47 mm Hg. For VD/VT the correlations were r = 0.95 and r2 = 0.91 (P < .001), and the bias and precision were 0.02 ± 0.04. The correlations between the ventilator and the volumetric CO2 monitor for PĒCO2 were r = 0.96 and r2 = 0.92 (P < .001), and the bias and precision were −0.19 ± 1.58 mm Hg. The correlations between the ventilator and the volumetric CO2 monitor for VD/VT were r = 0.97 and r2 = 0.95 (P < .001), and the bias and precision were 0.01 ± 0.03.
CONCLUSIONS: PĒCO2, and therefore VD/VT, can be accurately calculated directly from the Dräger XL ventilator volumetric capnography measurements without use of a metabolic analyzer or volumetric CO2 monitor.
- dead space fraction
- metabolic analyzer
- volumetric CO2 monitor
- ventilator
- volumetric capnography
- volumetric capnogram
Introduction
Dead-space ventilation, the portion of a tidal volume that does not contribute to gas exchange, was first described and calculated by the Bohr equation in 1891,1 and later by the Enghoff modification of the Bohr equation in 1938.2 Physiologic dead-space fraction (dead space divided by tidal volume [VD/VT]), as defined by Bohr and Enghoff, is the sum of anatomic or airway dead space (VD-anat) and alveolar dead space (VD-alv) divided by the VT. The definition of pure dead space is ventilation without perfusion, whereby alveolar gases do not contact blood flowing through the pulmonary capillaries. All conducting airways (anatomical and mechanical dead space), areas of pure shunt (pulmonary capillary perfusion with no ventilation), areas of pure dead space, and the presence of gas exchange units with any degree of inequality of ventilation in relation to perfusion, can contribute to the calculated dead-space ventilation.
Assessing VD/VT in critically ill patients during mechanical ventilation is important for several reasons.3 The prognostic value of VD/VT has been linked to mortality risk in ARDS4–7 and to other important clinical indices. VD/VT is known to correlate with the severity of lung injury,8–12 can be useful as an indicator of lung recruitment versus overdistention in patients with acute lung injury (ALI) and ARDS,13–17 may be helpful as a predictor of successful extubation in pediatric18 and adult patients,19 and may be useful in diagnosing and assessing the severity of pulmonary embolism.20,21
Simplified bedside calculation of VD/VT requires a measurement of the partial pressure of mean expired CO2 (PĒCO2) and use of the Enghoff modification of the Bohr equation.2 The Enghoff equation differs from the original Bohr equation by the substitution of PaCO2 for the partial pressure of mixed alveolar CO2 (PACO2). The Enghoff equation became the standard in clinical practice for calculation of VD/VT because PACO2 has been difficult to accurately measure or estimate at the bedside. The traditional technique of measuring PĒCO2 used the Douglas bag method of exhaled gas collection and analysis.22 Technological advancements allow the use of a metabolic analyzer,23,24 and, more recently, use of volumetric capnography and a volumetric CO2 monitor.25
The Dräger XL ventilator (Dräger Medical, Telford, Pennsylvania) is equipped with integrated CO2 and volume measurement capabilities (volumetric CO2). We calculated PĒCO2 and VD/VT directly from the Dräger XL ventilator volumetric CO2 measurements of V̄CO2 and compared them to metabolic analyzer and volumetric CO2 monitor measurements of PĒCO2 and VD/VT.
QUICK LOOK
Current knowledge
Measurement of the ratio of physiologic dead space (VD) to tidal volume (VT) with mixed expired carbon dioxide and blood gas analysis can be accomplished with various commercially available monitors. In acute respiratory distress syndrome, higher VD/VT is associated with higher mortality.
What this paper contributes to our knowledge
VD/VT was accurately measured by volumetric capnography on the Dräger XL ventilator, compared with 3 metabolic analyzers (Metascope, Deltatrac, and Vmax Encore) and the NICO monitor. Volumetric capnography on the Dräger XL ventilator obviates the use of a stand-alone analyzer for measuring VD/VT.
Methods
A total of 67 measurements were performed in 36 subjects who met the American-European Consensus Conference criteria for ALI or ARDS.26 Measurement were done at varying time periods after ALI or ARDS criteria were met (Table 1). Phase 1 of the study compared 31 ventilator derived measurements in 25 subjects to measurements from 3 different metabolic analyzers:, Metascope (Cybermedic, Louisville, Colorado, n = 9), Deltatrac (SensorMedics, Yorba Linda, California, n = 4), and Vmax Encore (Viasys, Yorba Linda, California, n = 18). Use of the various metabolic analyzers was based on functional availability. All metabolic analyzers used were maintained by annual biomedical engineering preventive maintenance and performance verification. In phase 2 of the study, 36 ventilator derived measurements in 11 subjects were compared to the NICO2 Respiratory Profile Monitor (Philips Healthcare, Andover, Massachusetts).
Subject Characteristics
VD/VT measurements were performed when requested by the ICU team. Arterial blood gas samples for PaCO2 determination and VD/VT calculation were obtained from arterial catheters. Prior to all measurements, ventilator, metabolic analyzer, and NICO2 monitor CO2 and flow sensors were calibrated using the manufacturers' specifications. Following all ventilator circuit disconnections, approximately 30 min was allowed for patient stabilization. Ventilator measurements were done simultaneously during metabolic analyzer and NICO2 monitor measurements, for comparison. The ventilator circuit was checked for leaks, and patients with active pulmonary air leaks were excluded from the study. The study was approved by the Committee on Human Research at the University of California, San Francisco.
Ventilator Volumetric CO2 Measurements
The Dräger XL ventilator's mainstream CO2 sensor was placed between the ventilator circuit and the patient connection. The ventilator expiratory flow sensor positioned at the distal side of the expiratory valve measured exhaled VT and exhaled minute ventilation (V̇E). Ventilator volumetric CO2 measurements were initiated and displayed on the ventilator trend data screen. After measured values stabilized and reached a steady state, ventilator trend data for minute CO2 production (V̇CO2) and V̇E were averaged over 5 min. All measurements were reported at body temperature, and pressure, saturated (BTPS).
The fraction of exhaled CO2 (FECO2) was calculated manually by dividing the ventilator derived V̇CO2 by the V̇E:
PĒCO2 was then calculated by multiplying FECO2 by the barometric pressure minus water vapor pressure:
PĒCO2 was then used to calculate VD/VT by the Enghoff modification of the Bohr equation:
The automated ventilator correction for delivered and measured VT was used by performing a circuit compliance test at device startup. The automated ventilator correction adjusts the delivered and measured VT, and therefore the ventilator calculated values for V̇CO2 and V̇E reflect the adjusted values and eliminate the need for a manual circuit compression volume correction.
Metabolic Analyzer Measurements
The metabolic analyzers were warmed up for 20 min and calibrated per the manufacturers' recommendations. The inspired gases were sampled from the ventilator's inspiratory limb, and the exhaled gases and volumes were measured by directing expiratory gas flow into the metabolic analyzer (Metascope and Deltatrac) or by placement of the metabolic analyzer flow sensor and expired gas sampling line at the ventilator expired gas outlet (Vmax Encore). After a stable 10-min measurement period, FECO2 averaged over a 5 min period from the metabolic analyzer was used to calculate PĒCO2, using equation 2 above.
All metabolic analyzer measurements of PĒCO2 were corrected for circuit compression volume, as previously described,23–25,27–29 whereby the PĒCO2 was multiplied by the ratio of the observed VT divided by the observed VT minus the calculated compression volume using the following equations:
Ventilator circuit compliance factors of 2.5 and 2.0 mL/cm H2O were used pre and post a ventilator circuit configuration change that was implemented during the study period. The circuit compliance factor of 2.5 mL/cm H2O was used for the Metascope and Deltatrac, and 2.0 mL/cm H2O was used for the Vmax Encore. Circuit compression volume was determined by laboratory testing and confirmed by the ventilator circuit compliance test mentioned above. VT was derived by dividing the V̇E by the breathing frequency measured by the metabolic analyzer. The Dräger XL ventilator uses a non-bias-flow triggering method, and therefore additional correction for potential measurement error caused by bias flow was unnecessary.
NICO2 Monitor Measurements
The NICO2 monitor combined CO2/flow sensor was allowed to warm up for 5 min until stable measurements for PĒCO2 were obtained. Both the NICO2 combined sensor and the ventilator mainstream CO2 sensor were placed between the ventilator circuit and the subject. The NICO2 sensor and the ventilator CO2 sensor were placed distal and proximal to each other in random order. In a previous bench study, the distal or proximal position of either sensor did not result in position related bias.30 PĒCO2 derived from the ventilator measurements was rounded to the nearest whole number for comparison to the NICO2 monitor display of PĒCO2.
Since the NICO2 combined CO2/flow sensor measures distal to the ventilator Y-piece, the effects of ventilator circuit compression volume and the utilization of a correction factor are unnecessary.
Statistical Analysis
The ventilator derived measurements of V̇CO2, FECO2, PĒCO2, and VD/VT were compared to the metabolic analyzer measurements. The PĒCO2 and VD/VT derived from ventilator measurements were compared to the NICO2 monitor measurements. The data were compared and analyzed by correlation measured by the Pearson product-moment correlation coefficient (r) and the coefficient of determination (r2). Bias and precision were assessed by Bland-Altman analysis. Statistical analysis was done using commercially available software (Excel, 14.2.2, Microsoft, Redmond, Washington). Correlation results were considered to be significant when P < .05.
Results
There was a strong correlation, agreement, and accuracy between the ventilator derived measurements and the metabolic analyzer or volumetric CO2 monitor measurements of V̇CO2, FECO2, PĒCO2, and VD/VT.
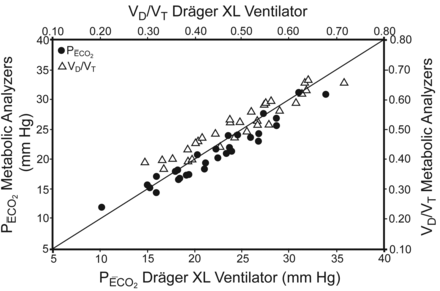
In phase 1 of the study, the correlations between the ventilator derived measurements and the metabolic analyzer measurements for V̇CO2 and FECO2 were r = 0.92 and r2 = 0.85 (P < .001), and r = 0.95 and r2 = 0.91 (P < .001). The bias and precision for V̇CO2 and FECO2 were 24 ± 31 mL/min and 0.07 ± 0.23%, respectively. The correlations for PĒCO2 were r = 0.97 and r2 = 0.93 (P < .001) (Fig. 1), and the bias and precision were −1.04 ± 1.47 mm Hg (Fig. 2). The correlations for VD/VT were r = 0.95 and r2 = 0.91 (P < .001) (see Fig. 1), and the bias and precision were 0.02 ± 0.04 (Fig. 3). The P value of 2 tailed probability for the Pearson correlation coefficient reached statistical significance for each metabolic analyzer for both PĒCO2 and VD/VT, except for one where the sample size was very small (DeltaTrac, n = 4, VD/VT, P = .09). Similarly, bias and precision remained within clinically acceptable ranges when individual measurements between the 3 metabolic analyzers were compared to the combined data from all 3 analyzers (Table 2).
Correlation of partial pressure of mean expired CO2 (PĒCO2) and ratio of dead space to tidal volume [VD/VT] between the Dräger XL ventilator and 3 different metabolic analyzers, plotted against the line of identity. For PĒCO2, r = 0.97 and r2 = 0.93 (P < .001). For VD/VT, r = 0.95 and r2 = 0.91 (P < .001).
Bland-Altman plot comparing partial pressure of mean expired CO2 (PĒCO2) calculated by measurements from the Dräger XL ventilator and 3 different metabolic analyzers. The bias and precision are −1.04 ± 1.47 mm Hg (95% CI −3.91 to 1.84 mm Hg).
Bland-Altman plot comparing the ratio of dead space to tidal volume [VD/VT], calculated by measurements from the Dräger XL ventilator and 3 different metabolic analyzers. The bias and precision are 0.02 ± 0.04 (95% CI −0.05 to 0.09).
Correlation, Bias, and Precision Between Individual Metabolic Analyzers, Compared to the Combined Data From All 3 Analyzers
In phase 2 of the study, the correlations, between the ventilator and the volumetric CO2 monitor measurements for PĒCO2 were r = 0.96 and r2 = 0.92 (P < .001) (Fig. 4), and the bias and precision were −0.19 ± 1.58 mm Hg (Fig. 5), and for VD/VT the correlations were r = 0.97 and r2 = 0.95 (P < .001) (see Fig. 4), and the bias and precision were 0.01 ± 0.03 (Fig. 6).
Correlation of partial pressure of mean expired CO2 (PĒCO2) and ratio of dead space to tidal volume [VD/VT] between the Dräger XL ventilator and the NICO2 volumetric CO2 monitor, plotted against the line of identity. For PĒCO2, r = 0.96 and r2 = 0.92 (P < .001). For VD/VT, r = 0.97 and r2 = 0.95 (P < .001).
Bland-Altman plot comparing partial pressure of mean expired CO2 (PĒCO2) calculated by measurements from the Dräger XL ventilator and the NICO2 volumetric CO2 monitor. The bias and precision are −0.19 ± 1.58 mm Hg (95% CI −3.30 to 2.91 mm Hg).
Bland-Altman plot comparing the ratio of dead space to tidal volume [VD/VT] calculated by measurements from the Dräger XL ventilator and the NICO2 volumetric CO2 monitor. The bias and precision are 0.01 ± 0.03 (95% CI 0.01–0.03).
Discussion
The results of this study confirm that PĒCO2, and therefore VD/VT, using the Enghoff equation can be accurately calculated directly from the Dräger XL ventilator's volumetric capnography measurements, without use of a metabolic analyzer or volumetric CO2 monitor. In a recent study, use of volumetric capnography calculations of VD/VT from the Dräger XL ventilator were shown to be a predictor of extubation success.19 However, to our knowledge the results of our study are the first to validate the accuracy of the Dräger XL measurements of VD/VT against previously accepted methods.
These findings have several important implications. This simplified approach to VD/VT measurement will improve availability, allow early and repeated measurements, and will increase the utilization of VD/VT for prognostic, diagnostic, and disease severity monitoring in the critical care setting. VD/VT has been shown to be predictive of the mortality risk in patients with ARDS in both the early and intermediate phases of the disease progression in single center or small cohort studies.4–8 Patients with VD/VT ≥ 0.57 were found to have higher mortality, with a 45% increase in the odds of dying for every 0.05 increase in dead-space fraction.5 VD/VT is also known to be a marker of the severity of lung injury.9–13 Serial monitoring of VD/VT over the duration and course of ALI/ARDS can be useful as a means to assess the need and effects of supportive therapeutic strategies and interventions.12,13 VD/VT measurements in patients with ALI/ARDS have been found to be useful for titrating PEEP and optimizing cardiopulmonary function,14–16 and may be useful as a tool to monitor lung recruitment versus overdistention.17 Assessment of VD/VT may also be used to predict successful extubation in pediatric18 and adult patients.19 VD/VT ≤ 0.50 and ≥ 0.65 in infants and children were found to be predictive of extubation success or failure,18 whereas in adult patients the VD/VT cutoff value that offered the best sensitivity and specificity for predicting extubation failure was 0.58.19 VD/VT, in addition to other clinical assessments and diagnostic tests, has also been used in diagnosing and assessing the severity of pulmonary embolism.20,21 The culmination of the broad clinical value of VD/VT assessments in the critical care setting support the integration of this measurement into routine clinical practice.
Additionally, use of a separate standalone device for VD/VT measurements, with the associated acquisition and supply costs and staff utilization time, may become unnecessary. Elimination of a metabolic analyzer or volumetric CO2 monitor simplifies the determination of VD/VT. Ventilator derived volumetric CO2 measurement makes physiologic dead-space fraction more accessible in clinical practice, as ventilator manufacturers incorporate volumetric CO2 monitoring capabilities into newer ventilator platforms.31 The calculations performed for his study were done manually, using ventilator derived measurements, but could easily be incorporated as an automated feature by ventilator software modification. Methods for estimating VD/VT by predictive equations have been described using the arterial to end-tidal CO2 difference32,33 and estimation of V̄CO2.34 The increasing availability of volumetric capnography make the use of predictive equations unnecessary.
The results of this study are consistent with prior data that confirm the accuracy of different methods of calculating VD/VT (Table 3). Similar correlation and accuracy of the exhaled gas collection method using a Douglas bag versus a metabolic analyzer,23,24 a metabolic analyzer versus a volumetric CO2 monitor,25 and ventilator based volumetric capnography versus a metabolic analyzer and a volumetric CO2 monitor are now demonstrated.
Correlation, Bias, and Precision From Studies That Compared Different Methods for Calculating the Ratio of Dead Space to Tidal Volume Using the Enghoff Equation
Limitations of this study include a relatively small sample size in each study phase, and the use of 3 different metabolic analyzers in phase 1. Also, the ventilator circuit configuration, and therefore the circuit compression volume, were changed during the study. Despite these factors, the resiliency of the ventilator derived data in relation to the correlation, bias and precision between measurements remained consistent. Although the number of individual measurements between the 3 metabolic analyzers used varied markedly, the agreement of correlation, bias, and precision remained consistent between individual metabolic analyzers, when compared to the combined data from all 3 analyzers (see Table 2). Additionally, the change in ventilator circuit configuration and compression volume did not significantly alter the correlation and agreement of the measurements (see Table 2).
VD/VT calculated by the original Bohr equation has been recognized as “true dead space” or the balance between effective and ineffective ventilation. The Bohr dead-space equation relies on the calculation or estimation of PACO2 from mixed alveolar gas. PACO2 is affected by the dilution of CO2 from the alveolar side of the alveolar-capillary membrane before the effects of shunt and venous admixture on PaCO2. Bohr dead space is affected by areas of high ventilation to perfusion matching, such as alveolar overdistention by excessive PEEP and/or VT, pulmonary vascular occlusion, and pulmonary hypoperfusion secondary to hypovolemia.35 The Enghoff equation, on the other hand, relies on the PaCO2 of arterial blood and is thus an index of “true dead space” plus the effects of elevated PaCO2 from global gas exchange inefficiency and shunt (Fig. 7). Elevated PaCO2 can result from all causes of low ventilation/perfusion matching and shunt, such as atelectasis, pneumonia, COPD, and asthma. Furthermore, PaCO2 can rise when an increase in metabolic rate and CO2 production are not accompanied by an increase in CO2 excretion. Changes in PaCO2 are determined by the relationship between V̇CO2 and minute alveolar ventilation (V̇A) whereby:
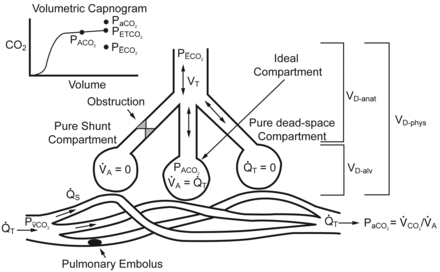
The 3 compartment lung model described by Riley36,37 represents gas exchange in the lung in regards to the matching of alveolar ventilation (V̇A) and perfusion (Q̇T), shunt (Q̇S), and dead space (VD). The ideal compartment represents areas of perfect V̇A to QT matching. The pure shunt compartment represents areas of perfusion without ventilation. The pure dead-space compartment represents areas of ventilation with no perfusion. The sum of the regions of alveolar dead space (VD-alv) and anatomic dead space (VD-anat) equal the physiologic dead space (VD-phys). Dead space fraction is equal to VD-phys divided by tidal volume (VT). Also shown are the partial pressure of arterial carbon dioxide (PaCO2), the partial pressure of venous carbon dioxide (PV̄CO2), the relationship between PaCO2 and minute CO2 production (V̇CO2) and V̇A, the partial pressure of mixed alveolar carbon dioxide (PACO2), the partial pressure of end-tidal carbon dioxide (PETCO2), and the partial pressure of mean expired carbon dioxide (PĒCO2) in relation to the model and the volumetric capnogram. (From references 35 and 38, with permission.)
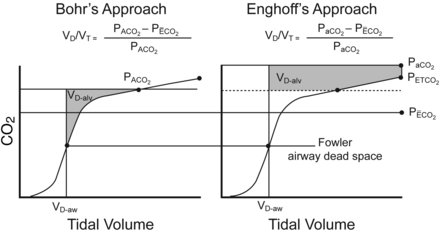
If V̇CO2 increases without a proportional rise in V̇A, CO2 production exceeds CO2 excretion and PaCO2 increases. Therefore the Enghoff dead-space equation can overestimate VD/VT in the presence of shunt and regions of low ventilation/perfusion ratio (Fig. 8).
Graphical representation of physiologic dead space fraction determined by volumetric capnography, using the approaches of Bohr and Enghoff, which shows how use of the Enghoff equation can overestimate alveolar dead space (VD-alv) (shaded areas) by substitution of the partial pressure of arterial carbon dioxide (PaCO2) for the partial pressure of mixed alveolar carbon dioxide (PACO2), determined by identifying the mid-point of phase III of the expired volumetric capnogram.39,40 Also shown are the airway or anatomical dead space (VD-aw, determined by the Fowler method identified at the mid-point of phase II of the expired volumetric capnogram,41), the partial pressure of end-tidal carbon dioxide (PETCO2), and the partial pressure of mean expired carbon dioxide (PĒCO2) in relation to the volumetric capnogram. (From reference 35, with permission.)
Use of volumetric capnography to determine PACO2 for Bohr dead-space calculation has been demonstrated39 and validated in an animal model of lung injury.40 PACO2 measured at the mid-point of phase III of the expired volume capnogram was compared to the PACO2 mathematically derived using the multiple inert gas elimination technique (MIGET). There was a close linear correlation between the 2 methods for calculating PACO2 (r = 0.99, P < .001) and Bohr dead space (r = 0.96, P < .001). The mean PACO2 and Bohr dead space from volumetric capnography were similar to the calculations obtained by MIGET, with a mean bias of −0.10 mm Hg (95% CI −2.18 to 1.98 mm Hg) and 10 mL (95% CI −44 to 64 mL), respectively. Given these findings, it has been suggested that simultaneous assessment of Bohr and Enghoff dead space using volumetric capnography may provide useful complementary information in regard to recognizing the effects of shunt and ventilation/perfusion inequality versus “true dead space” or wasted ventilation in critically ill patients with elevated VD/VT.35 Furthermore, using volumetric capnography to determine Bohr dead space could be monitored continuously and would not require periodic arterial blood sampling to measure PaCO2.35
Conclusions
This study confirms that PĒCO2 and VD/VT using the Enghoff equation can be accurately calculated directly from the Dräger XL ventilator's volumetric capnography measurements, without use of a metabolic analyzer or volumetric CO2 monitor.
Future study of the use and development of ventilator based techniques for volumetric capnography measurements of VD/VT should continue to confirm and validate measurement correlation and accuracy. Investigation of the meaningful use of continuous Bohr VD/VT monitoring and simultaneous measurement of Bohr and Enghoff VD/VT using volumetric capnography should also be pursued.
Footnotes
- Correspondence: Mark S Siobal RRT FAARC, Respiratory Care Services, San Francisco General Hospital, 1001 Potrero Avenue, NH GA2, San Francisco CA 94110. E-mail: msiobal{at}sfghsom.ucsf.edu.
The authors have disclosed no conflicts of interest.
Mr Siobal presented a version of this paper at the 56th AARC Congress, held December 6–9, 2010, in Las Vegas, Nevada.
See the Related Editorial on Page 1258
- Copyright © 2013 by Daedalus Enterprises