Abstract
BACKGROUND: Patient-triggered adaptive pressure control (APC) continuous mandatory ventilation (CMV) (APC-CMV) has been widely adopted as an alternative ventilator mode to patient-triggered volume control (VC) CMV (VC-CMV). However, the comparative effectiveness of the 2 ventilator modes remains uncertain. We sought to explore clinical and implementation factors pertinent to a future definitive randomized controlled trial assessing APC-CMV versus VC-CMV as an initial ventilator mode strategy. The research objectives in our pilot trial tested clinician adherence and explored clinical outcomes.
METHODS: In a single-center pragmatic sequential cluster crossover pilot trial, we enrolled all eligible adults with acute respiratory failure requiring mechanical ventilation admitted during a 9-week period to the medical ICU. Two-week time epochs were assigned a priori in which subjects received either APC-CMV or VC-CMV The primary outcome of the trial was feasibility, defined as 80% of subjects receiving the assigned mode within 1 h of initiation of ICU ventilation. The secondary outcome was proportion of the first 24 h on the assigned mode. Finally, we surveyed clinician stakeholders to understand potential facilitators and barriers to conducting a definitive randomized trial.
RESULTS: We enrolled 137 subjects who received 152 discreet episodes of mechanical ventilation during time epochs assigned to APC-CMV (n = 61) and VC-CMV (n = 91). One hundred and thirty-one episodes were included in the prespecified primary outcome. One hundred and twenty-six (96%) received the assigned mode within the first hour of ICU admission (60 of 61 subjects assigned APC-CMV and 66 of 70 assigned VC-CMV). VC-CMV subjects spent a lower proportion of first 24 h (84% [95% CI 78–89]) on the assigned mode than APC-CMV recipients (95% [95% CI 91–100]). Mixed-methods analyses identified preconceived perceptions of subject comfort by clinicians and need for real-time education to address this concern.
CONCLUSIONS: In this pilot pragmatic, sequential crossover trial, unit-wide allocation to a ventilator mode was feasible and acceptable to clinicians.
Introduction
Volume-targeted modes of mechanical ventilation are the mainstay of ventilator support in critically ill patients requiring tracheal intubation.1 Historically, volume control (VC) continuous mandatory ventilation (CMV) (VC-CMV) was the most commonly used volume-targeted mode; however, additional modes of ventilation such as adaptive pressure control (APC) CMV (APC-CMV) have been incorporated into modern ventilators as alternatives.2 Whereas VC-CMV delivers a set tidal volume each breath, APC-CMV delivers an algorithmically determined inspiratory pressure predicted to deliver an ordered tidal volume.3 Because these algorithms are proprietary, APC-CMV is branded using manufacturer-specific names such as pressure-regulated VC and AutoFlow. APC-CMV has been marketed to clinicians as a dynamic mode that controls both pressure and volume while responding to changes in respiratory system compliance.4 APC-CMV use has increased, becoming the default mode of mechanical ventilation at some institutions.2,5 Despite the ubiquitous use of mechanical ventilation in the ICU, few data are available to inform the selection of volume-targeted ventilator modes. Limited observational data suggest that APC-CMV may reduce ventilator asynchrony compared to VC-CMV.6 However, in approximately 10% of patients, APC-CMV delivers larger-than-ordered tidal volumes that are outside of the lung-protective range.7 Few experimental models or clinical trials have compared VC-CMV to APC-CMV, and whether ventilator mode selection impacts tidal volume delivery and clinical outcomes is unknown.8,9 As both APC-CMV and VC-CMV are accepted as usual care, a pragmatic comparative effectiveness trial is an appropriate way to compare these modes. However, factors including feasibility, clinician preference, and acceptability may impact successful trial conduct. We designed a pragmatic sequential crossover pilot study to investigate these factors as well as relevant clinical outcomes. The aims of the current trial were to test feasibility and identify potential implementation facilitators and barriers relevant for a definitive randomized controlled trial (RCT) comparing APC-CMV versus VC-CMV and initial ventilator mode in mechanically ventilated medical ICU (MICU) subjects.
QUICK LOOK
Current knowledge
Most patients with acute respiratory failure receive mechanical ventilation using one of 2 volume-targeted modes: volume control (VC) continuous mandatory ventilation CMV (VC-CMV) or adaptive pressure control CMV (APC-CMV). APC-CMV may reduce ventilator asynchrony but is associated with a risk of excessively large tidal volumes in approximately one in 10 patients.
What this paper contributes to our knowledge
In a pragmatic, sequential crossover trial, we found that unit-wide allocation to either VC-CMV or APC-CMV was both feasible and acceptable to clinicians. Subjects assigned to VC-CMV were more likely to be switched to an alternative mode in the first 24 h of ventilation.
Methods
Study Design and Oversight
The Pragmatic Investigation of Volume Targeted Ventilation-1 (PIVOT-1) study was a pragmatic single-center, unblinded, sequential cluster crossover feasibility pilot trial that evaluated adherence to allocated ventilator modes (VC-CMV and APC-CMV). The trial was approved by the Wake Forest Institutional Review Board (IRB00055128) with a waiver of informed consent and was registered online prior to initiation (NCT03909854). Funding for PIVOT-1 was provided by the Wake Forest Critical Illness, Injury, and Recovery Research Center.
Trial Population
We conducted the trial from September 10, 2019–November 9, 2019, in the Wake Forest medical ICU (MICU), a tertiary academic closed ICU staffed by intensivists overseeing teams of pulmonary and critical care fellows, house staff, advanced practice providers, and critical care–trained respiratory therapists. The protocolized baseline ventilator mode for clinical care in this ICU is APC-CMV; however, alternative modes are used at clinician's discretion. The trial population comprised consecutive adults (age ≥ 18 y) admitted to the MICU requiring invasive mechanical ventilation.
Subject Identification
We used ventilator orders in the electronic health record (EHR) to capture subjects receiving mechanical ventilation. For this pilot study, we confirmed receipt by EHR review and, when available, Health Level Seven (HL7) messaging (a standardized clinical data messaging language).10 Subjects intubated in the emergency department (ED), hospital ward, or at outside hospitals were included. Subjects receiving chronic mechanical ventilation via tracheostomy or initially managed with a non–volume-targeted mode as the initial mode of mechanical ventilation (eg, pressure support) due to clinician's choice were excluded. Enrolled subjects were eligible to participate again if, after their initial extubation, they required re-intubation and mechanical ventilation in the MICU.
Treatment Assignments
The trial protocol guided the mode of ventilator support that was initiated in the MICU. To maximize pragmatism, the interventions were delivered by clinical personnel rather the members of a dedicated study team. All aspects of care other than mode of ventilator support including ventilator settings, sedation, and weaning approach were determined by treating clinicians independent of the trial protocol.
The initial mode of ventilatory support was assigned according to epoch; the first trial epoch was assigned to APC-CMV starting on September 10, 2019, because that was the current standard practice in the study MICU prior to study initiation. Treatment assignments then sequentially crossed over to VC-CMV after 2 weeks and, after 5 weeks of VC-CMV allocation, back to APC-CMV for a total of 9 weeks of enrollment (supplement Fig. 1, see related supplementary materials at http://www.rcjournal.com). Because APC-CMV was already the familiar ventilator mode in the study MICU, additional education was provided on VC-CMV use prior to and during the epoch allocated to VC-CMV. Clinicians were aware of the treatment assignments. Selection of ventilator mode after 24 h was not part of the trial intervention.
Flow chart.
Implementation Strategies to Encourage Adherence to Assigned Intervention
Members of the study team informed ICU clinicians about the trial and provided training on VC-CMV ventilator asynchrony diagnosis and management (supplemental Fig. 2, see related supplementary materials at http://www.rcjournal.com). At the study start and on mode switch weeks, the lead investigator attended the morning ICU provider huddle to refresh clinicians on study protocols and treatment assignment. The respiratory therapist investigator provided refresher training at study launch and mode switch weeks at the respiratory therapy huddle. All subjects received APC-CMV (CMV + AutoFlow) or VC-CMV (Dräger CMV) via Dräger Evita 4, Evita 2 dura, Evita XL, or V500 ventilators (Dräger, Lübeck, Germany). Clinicians were encouraged to start the assigned mode as soon as possible after initiation of ICU mechanical ventilation. In subjects intubated outside of the ICU (in the ED, hospital ward, or outside hospital), the ventilator was set to the assigned mode upon arrival in the study ICU. Clinicians could order off-protocol ventilator modes any time they believed an alternative to the assigned mode was appropriate. The study team met with key clinician stakeholders during week 5 of the study to review implementation barriers. No major barriers were identified at this meeting, and the study proceeded without modification.
Embedded Implementation Evaluation Procedures
To gain additional input on potential facilitators and barriers to a future large-scale pragmatic RCT, we surveyed a diverse group of clinician stakeholders including intensivists, respiratory therapists, and pulmonary and critical care fellows. The survey was created by our study team in REDCap and pilot tested prior to dissemination (supplementary material, see related supplementary materials at http://www.rcjournal.com).11 We used a Likert scale to assess volume-targeted mode acceptability and also assessed feasibility of conducting a full RCT. Additionally, we included 3 open-ended questions to understand the preferred mode of mechanical ventilation and strategies to improve adherence to mode assignment and education. The survey was distributed to all fellows, faculty, and respiratory therapists at regularly scheduled staff meetings and through e-mail.
Data Collection
For enrolled subjects, the study began at initiation of mechanical ventilation in the MICU and ended at study termination (which occurred at 90 d after enrollment or death). Data were electronically captured from the medical record at enrollment, daily while receiving mechanical ventilation, and at 90 d post enrollment. Collected data included baseline demographics, comorbidities, indication for mechanical ventilation, mode of mechanical ventilation, Richmond Agitation-Sedation Scale (RASS) scores, agitation and sedation index over first 48 h, number of sedation infusions, highest Sequential Organ Failure Assessment score on ICU day 1, shock (defined as receipt of vasopressors), receipt of cisatracurium infusion, and diagnosis of ARDS within 48 h of ICU ventilation12–14 Ventilator mode over the first 24 h was manually confirmed by study staff from the electronic medical record ventilator flow sheet. ARDS was defined using the Berlin criteria. Each subject's chest imaging was independently reviewed by 2 study physicians to determine if they met Berlin ARDS radiographic criteria. In the event of reviewer discordance, a third study physician adjudicated the chest imaging. Additional data collected included 28 ventilator-free days, ICU stay, hospital stay, and vital status at 90 d.15
Ventilator parameters were recorded using the Capsule Neuron data router system (Philips, Amsterdam, the Netherlands) and transmitted via HL7 messages (iNTERFACEWARE, Toronto, Ontario, Canada). HL7 messages were generated every minute and detected by a Mirth Connect HL7 receiver. Minute-by-minute tidal volume data were collected in all subjects enrolled between October 7, 2019, and end of study.10 Tidal volume data were analyzed if at least 60 min of data were available.
Study Outcomes
The primary outcome of the trial was adherence to assigned ventilator mode within 1 h of initiation of ICU mechanical ventilation, with a predefined feasibility threshold of 80% of subjects receiving the assigned mode within 1 h of initiation. A secondary feasibility outcome was > 70% of time on the assigned mode during the first 24 h of mechanical ventilation (excluding time spent on spontaneous breathing trials and censored at extubation or death). We chose to monitor adherence during the first 24 h as limited data suggest that early mechanical ventilation may disproportionately affect outcomes.16 Seventy percent was selected as this secondary feasibility cutoff based on a recent RCT that found that approximately 50% of subjects randomized to volume-targeted ventilation were transitioned to a non–volume-targeted mode on day 1.17 Based on these data, we conservatively estimated 30% of subjects enrolled in PIVOT-1 would transition to a non-VC mode by day 2 of mechanical ventilation and that this transition would be consistent with standard-of-care mechanical ventilation. Clinical outcomes collected included ventilator-free days, ICU stay, hospital stay, depth of sedation, number of sedation infusions, and 90-d mortality.
Statistical Analysis
The primary outcome analysis excluded subjects enrolled during the washout week. Two subjects were enrolled in both arms and included in the feasibility outcomes but excluded from the secondary and exploratory analyses as they were assigned to both APC-CMV and VC-CMV. All analyses were conducted as intention to treat. Continuous variables were reported as median and interquartile range and categorical variables as frequencies and proportions. Confidence intervals for frequencies were calculated as exact binomials. Between-group comparisons were made with Kruskal-Wallis or Wilcoxon rank-sum tests for continuous variables or Fisher exact test for categorical variables. For clinician survey data, 5-point Likert scale responses were analyzed using mode and mean. Between-group comparisons of survey data were compared using Wilcoxon rank-sum tests. For analysis of open-ended survey responses, 2 study investigators (JP, KG) provided independent open-ended review of text fields to elicit themes for why clinicians may choose a particular mode and any barriers or facilitators that may impact willingness to enroll in a larger trial. The 2 investigators met to discuss the emerging themes after initial review and provide input on final analysis included. Quantitative analyses were performed using R software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Pilot Feasibility Results
There were total of 169 episodes of mechanical ventilation identified during the study period (Fig. 1). After exclusions, 137 unique subjects during 152 episodes of ICU mechanical ventilation were assigned to APC-CMV (n = 61) or VC-CMV (n = 91). Subject characteristics including demographics, comorbidities, mechanical ventilation indication, severity of illness, and rate and severity of ARDS were similar between groups (Table 1). Twenty-one subjects received new mechanical ventilation during the washout week and were excluded from the feasibility end point. One hundred thirty-one episodes of mechanical ventilation were included in the primary end point. One hundred twenty-six of 131 subjects received the assigned mode within the first hour of ICU mechanical ventilation: 60 of 61 subjects (98% [95% CI 91–100]) and 66 of 70 subjects (94% [95% CI 86–98]) in the APC and assist VC groups, respectively, received the assigned mode as allocated. (Table 2). In a sensitivity analysis including episodes of mechanical ventilation during the washout week, adherence to the assigned mode also exceeded 80% (98% [95% CI 91–100] and 91% [95% CI 84–95] in APC-CMV and VC-CMV, respectively).
Baseline Subject Characteristics
Primary and Exploratory Outcomes
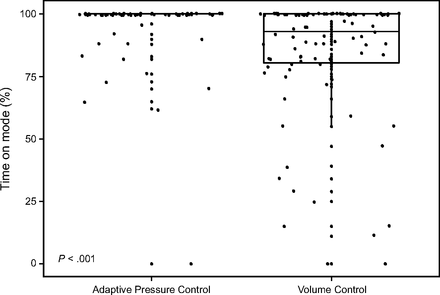
Both groups achieved the secondary feasibility target of 70% of time spent on the assigned ventilator mode over the first 24 h. Subjects assigned to APC-CMV spent 95% of the first 24 h (95% CI 91–98) on the assigned mode compared to 84% (95% CI 78–89) in the VC-CMV group (Fig. 2, Table 2). Duration of eligible day 1 h, duration of ventilation on assigned mode, and final mode of mechanical ventilation on day 1 are reported in supplemental Table 1 (See related supplementary materials at http://www.rcjournal.com).
Percent of eligible hours spent on the assigned mode on study day 1.
Exploratory clinical outcomes are also shown in Table 2. There was no difference in outcomes between groups in sedation index, agitation index, ventilator-free days, ICU length of stay, hospital length of stay, and 90-d mortality. Number of sedation infusions per day and depth of sedation as measured by RASS were also similar between groups. Starting on October 7, 2019, we analyzed HL7 ventilator data from approximately 68,000 minute-by-minute breaths in 67 subjects on study day 1. Twenty-seven episodes of APC-CMV and 40 episodes of VC-CMV were analyzed (Fig. 3). We compared the difference between ordered and exhaled tidal volume in subjects receiving APC-CMV and VC-CMV. Two of 27 subjects (7%) receiving APC-CMV had a median exhaled tidal volume > 1 mL/kg ideal body weight larger than ordered. None of the 40 subjects receiving VC-CMV had a median exhaled tidal volume 1 mL/kg ideal body weight larger than ordered. Minute-by-minute tidal volume data beyond day 1 are reported in the supplement (See related supplementary materials at http://www.rcjournal.com).
Ridge plots of the difference between exhaled and ordered tidal volume in subjects on adaptive pressure control continuous mandatory ventilation (CMV) and volume control CMV. Each ridge represents all recorded breaths for an individual subject while on volume-targeted mode on study day 1. IBW = ideal body weight.
Survey Results
The survey was distributed to 109 clinicians in the MICU, and 52 other clinicians (7 pulmonary and critical care fellows, 18 attending ICU physicians, and 27 respiratory therapists) responded (48% response rate). Providers were comfortable managing subjects using either VC-CMV or APC-CMV (Likert scale mode/mean 5/4.5 and 5/4.6, respectively). Thirty-eight (73%) respondents had a preferred initial mode of mechanical ventilation, with the majority (55%) preferring APC-CMV. Respondents provided the following reasons for their preferred mode: personal familiarity (n = 15), patient comfort (n = 8), and interpretation of the medical literature (n = 10). ICU attendings were more likely to prefer VC-CMV as an initial mode than fellows or respiratory therapists (P < .001). Despite most clinicians expressing a personal preference for one mode over the other, 49 of the 52 respondents (94%) would feel comfortable enrolling subjects in a larger definitive RCT in which subjects had equal likelihood of being assigned to VC-CMV or APC-CMV.
The following 3 themes (with representative quotations) were elicited after review of open-ended text fields:
Clinicians emphasize the importance of patient-centered decision making when selecting modes of ventilation:
“We need to treat patient[s] with the best mode for them.”
“I believe a trial is okay… sometimes patients don't respond well and we have to make adjustments either way.”
Clinicians perceive that VC-CMV may be uncomfortable for patients:
“Patients get very uncomfortable on volume control with light sedation/RASS, often times peak pressures become excessive.”
“I do not like to use volume control since flow needs are dynamic.”
Education and adherence strategies should be multimodal and real time:
“I think this depends if cluster of individual level randomization. If individual, suggest sign in room and note in EPIC. If cluster, morning huddle and likely signs posted around MICU would be better I think. Emails are last resort. ”
Respondents identified the following as key education and training components needed prior to a larger comparative effectiveness trial: ventilator simulator mode training (n = 34), ventilator didactics (n = 32), and ventilator asynchrony cards (n = 34).
Discussion
In this pragmatic pilot trial, unit-based assignment of volume-targeted ventilator mode resulted in clinician adherence to the assigned mode at the initiation and over the first 24 h of ICU mechanical ventilation.
In addition, a multidisciplinary group of clinician stakeholders expressed comfort with enrolling in a definitive RCT. These findings support the feasibility of a larger comparative effectiveness trial and will help inform its design and conduct.
Many studies have evaluated the effects of tidal volume size and mechanical ventilation on outcomes in critically ill subjects. Robust data show that delivering lung-protective tidal volumes (4–8 mL/kg ideal body weight) by mechanical ventilator reduces mortality in ARDS.18 However, the benefits of low tidal volume ventilation are much less clear in subjects without ARDS.5,17 Evidence suggests that low tidal volume has been widely adopted by clinicians for all critically ill subjects requiring mechanical ventilation.19 Our group and others have shown that APC-CMV results in excessively large tidal volumes in a subset of spontaneously breathing subjects.7,20 However, limited data also suggest that APC-CMV may reduce asynchrony.6 Excess tidal volume delivery may also occur in VC-CMV due to breath-stacking during double triggering.21 Despite the importance of controlling tidal volume, little evidence exists to inform the selection of a volume-targeted ventilator mode.8
In addition to confirming feasibility, our study had several key findings. At an institution where the routinely used mode is APC-CMV, subjects receiving VC-CMV were more likely to be switched to an alternative mode of mechanical ventilation on day 1. Our study did not demonstrate a difference in ventilator-free days, hospital or ICU stay, or mortality but was not powered to detect such differences.
Our survey of multidisciplinary clinician stakeholders found that clinicians consider randomization of volume-targeted ventilator mode acceptable. We identified concerns about patient comfort and patient-centered decision making as potential barriers to trial conduct. To address these concerns, pretrial education focusing on optimizing comfort and minimizing asynchrony is needed. Education should be multimodal and recurrent. Our trial has several strengths. We assigned ventilator mode at the ICU unit level, facilitating enrollment as early as possible in the individual subject's ventilator course. Additionally, our broad enrollment criteria reduced selection bias and confirmed that a pragmatic ventilator trial is possible in a diverse ICU subject population. We used minute-by-minute longitudinal tidal volume data to both assess for differences in delivered tidal volume and to test feasibility of delivered tidal volume as a secondary outcome in a larger trial.
The trial also has weaknesses. The study was a single-center pilot and, therefore, not intended to evaluate effectiveness. Treating clinicians and investigators were aware of group assignment, and although allocation blocks were assigned a priori, they were not randomized. APC-CMV was the protocolized default mode in the study ICU, which may influence clinician adherence and preferences. Although compliance with mode assignment met both feasibility criteria, a higher percentage of subjects in the VC-CMV group crossed over to an alternative mode of mechanical ventilation at the end of day 1 compared to the APC-CMV group, increasing the risk for contamination between groups. Additionally, all subjects received mechanical ventilation via Dräger ventilators, and the effect of APC-CMV on tidal volume may be brand specific. Finally, interpretation of survey data may be limited by selection bias.
Conclusions
Unit-based ventilator assignment and sequential crossover to volume-targeted ventilator modes are feasible and resulted in high clinician adherence to the assigned mode. Future research is needed to inform the selection of volume-targeted modes in critically ill subjects with respiratory failure.
Footnotes
- Correspondence: Kevin W Gibbs MD, 2nd floor Watlington Hall, Section on Pulmonary, Critical Care, Allergy, and Immunology, Medical Center Boulevard, Winston-Salem, NC 27157. E-mail: kgibbs{at}wakehealth.edu
Wake Forest Critical Illness, Injury, and Recovery Research Center provided funding for this trial. The work was also supported by K23 AG073529-01 (Dr Palakshappa).
Supplementary material related to this paper is available at http://www.rcjournal.com.
Dr Files discloses relationships with Medpace, CytoVale, and Global Blood Therapeutics. The remaining authors have disclosed no conflicts of interest.
See the Related Editorial on Page 1473
- Copyright © 2023 by Daedalus Enterprises