Abstract
BACKGROUND: Poor sleep quality is often reported among patients with COPD. Pulmonary rehabilitation (PR) is beneficial in improving exercise capacity and health-related quality of life (HRQOL). However, its benefit in terms of sleep quality in patients with COPD remains unclear. This study aimed to investigate the effects of PR on sleep quality of patients with COPD.
METHODS: Thirty-four subjects with COPD were studied. All subjects participated in a 12-week (2 sessions/week) hospital-based out-patient PR study. Baseline and post-PR status were evaluated by spirometry, a sleep questionnaire (Pittsburgh Sleep Quality Index [PSQI]), a disease-specific questionnaire of HRQOL (St George Respiratory Questionnaire [SGRQ]), cardiopulmonary exercise testing, respiratory muscle strength, and the Borg dyspnea scale.
RESULTS: Mean FEV1/FVC in the subjects was 0.49 ± 0.13, and the mean FEV1 was 1.06 ± 0.49 L/min (49.7 ± 18.0% of predicted). After PR, the PSQI score decreased from 9.41 ± 4.33 to 7.82 ± 3.90 (P < .001). The number of subjects with a PSQI score > 5 also decreased (85.3–64.7%, P = .006). There were significant improvements in HRQOL (SGRQ, P = .003), exercise capacity (peak oxygen uptake, P < .001; and work rate, P < .001), dyspnea score (P < .001), and respiratory muscle strength (inspiratory muscle strength, P = .005; and expiratory muscle strength, P = .004) after PR. There were no significant changes in pulmonary function test results (FEV1, P = .77; FVC, P = .90; FEV1/FVC, P = .90).
CONCLUSIONS: PR results in significant improvement in sleep quality, along with concurrent improvements in HRQOL and exercise capacity. PR is an effective nonpharmacologic treatment to improve sleep quality in patients with COPD and should be part of their clinical management.
Introduction
Poor sleep quality is often reported among patients with COPD.1 They frequently complain of difficulty initiating and maintaining sleep and an increased number of arousals during sleep.2 A previous polysomnographic study demonstrated low total sleep time, sleep fragmentation, and reduced amounts of deep sleep in patients with COPD.1 In previous studies, 61–75% of patients with COPD had poor sleep quality.2–4 Another study suggested that poor sleep quality happens in these patients regardless of Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification of COPD.4 Even those with mild-to-moderate COPD have poor sleep quality.5 However, poor sleep quality is frequently ignored by physicians in the management of COPD. Despite the apparent high prevalence of nighttime symptoms and disturbed sleep, there are only a few interventional studies that have directly targeted them.
Sleep is an important aspect of maintaining the body's circadian rhythm. Inadequate sleep has impact on long-term health consequences, and sleep disturbance is associated with cardiovascular risk factor abnormalities, type 2 diabetes mellitus, fatigue, lethargy, depression, impaired cognition, and overall poor quality of life.2,6–8 Because poor sleep quality contributes to the increased comorbidities and impact on general health, there should be increased efforts to treat poor sleep quality in COPD. Hypnotics should be avoided because of a potential deleterious effects on respiration during sleep.9 Instead, the management of sleep problems in COPD should first focus on optimizing the patient's overall respiratory condition. Previous studies showed equivocal benefits of theophylline, inhaled bronchodilators, and supplemental oxygen on sleep quality.10–15 Management should therefore not just concentrate on drug control of airway obstruction.
Subjective sleep quality can be assessed via questionnaire, clinical interviews, and sleep diaries,16 whereas objective sleep quality can be obtained by polysomnography and actigraphy.16 Although overnight in-laboratory polysomnography is the accepted standard for diagnosing sleep disorders, it is expensive and burdensome for patients. Furthermore, in-laboratory polysomnography may not reflect usual sleep conditions. Sleep is largely subjective in nature. Subjective sleep quality as assessed by a questionnaire provides useful insights into the patient's perception of the nocturnal burden. In a previous study, symptoms of depression were strongly associated with subjective sleep quality and only moderately associated with objective sleep quality.17 In the current study, the Pittsburgh Sleep Quality Index (PSQI) was used to evaluate subjective sleep quality in patients with COPD.
Pulmonary rehabilitation (PR) is often recommended as an integral part of management,18,19 and many studies confirm that with PR there is improvement in exercise capacity and health-related quality of life (HRQOL) in patients with COPD.20–22 Despite numerous studies confirming that PR improves HRQOL and exercise capacity, its benefit in sleep quality remains unclear. Nonetheless, PR is beneficial in improving skeletal and respiratory muscle strength,21,23 reduced dynamic hyperinflation,24 and adaptations in breathing patterns.25 Thus, PR may be considered beneficial to the sleep quality of patients with COPD.
Unfortunately, there are very few studies regarding PR and sleep quality in patients with COPD. The present study aims to investigate the benefits of PR in sleep quality in COPD. The primary objective was to determine the effect of PR on subjective sleep quality. The secondary objective was to determine the benefits of PR on HRQOL and exercise capacity.
QUICK LOOK
Current knowledge
Pulmonary rehabilitation (PR) is a comprehensive intervention including education, training, and behavior modification aimed at improving quality of life in patients with chronic respiratory disease. The impact of PR on sleep quality is not well described.
What this paper contributes to our knowledge
In a small group of subjects with COPD, a 12-week session of PR was associated with improvements in sleep quality as measured by a sleep questionnaire. Health-related quality of life and dyspnea were improved after PR.
Methods
Patient Selection
From May 2011 to November 2012, 34 subjects were recruited from the out-patient clinic of the Taipei Tzu Chi Hospital. The inclusion criteria were (1) a diagnosis of COPD based on the GOLD guidelines26; (2) stability with no exacerbations or worsening of respiratory symptoms, no increased use of rescue medication, and no unscheduled visits for at least 3 months27; and (3) ability to mobilize independently. The exclusion criteria were (1) history of other lung diseases such as pneumoconiosis, bronchiectasis, pulmonary tuberculosis, primary pulmonary hypertension, pulmonary embolism, and interstitial lung disease and (2) orthopedic, neurologic, or cardiovascular impairment that might render the subjects incapable of completing the exercise training. The research protocol was approved by the ethics committee of the Taipei Tzu Chi Hospital, and all subjects provided informed consent.
Measurements
Physiologic parameters were assessed by spirometry, respiratory muscle strength testing (maximum inspiratory pressure [PImax] and maximum expiratory pressure [PEmax]), and cardiopulmonary exercise testing. Sleep quality was assessed by the PSQI. The HRQOL and dyspnea symptoms were assessed using the St George Respiratory Questionnaire (SGRQ) and the Borg dyspnea scale. These assessments were performed before and after PR. The physician and technician who performed or interpreted these pre-PR and post-PR intervention measurements were not involved in the trial and were blinded to this study.
Sleep Quality Assessment
The validated Chinese version of the PSQI was used to measure sleep quality.28,29 The scale is composed of 19 items divided into 7 components: sleep quality, latency, duration, disturbances, habitual sleep efficiency, use of sleep medications, and daytime dysfunction. Each item is rated on a scale of 0–3, where zero indicates no difficulty, and 3 indicates severe difficulty. A global sleep quality index was calculated with scores ranging from 0 and 21.28,29 Higher PSQI scores represent worse sleep quality, and a PSQI score > 5 indicates poor sleep quality.28,29
Health-Related Quality of Life Assessment
The HRQOL was assessed using the validated Chinese version of the SGRQ,30 which is a questionnaire designed to measure the influence of chest diseases on HRQOL.30 Responses to its 50 items were aggregated into an overall score and 3 subscores for symptoms (8 items), activity (16 items), and impact (26 items). Responses were weighted, and scores were calculated by dividing the summed weights by the maximum possible weight, with zero as the best possible score and 100 the worst.30
Pulmonary Function Test
Pulmonary function testing for FEV1 and FVC were performed by spirometry (Medical Graphics, Minneapolis, Minnesota) following the standards of the American Thoracic Society and the European Respiratory Society.31,32 The best flow-volume loop was used in the final data analysis.
Respiratory Muscle Strength
The PImax and PEmax were assessed using a standard mouthpiece and direct-dial pressure gauge (respiratory pressure meter, CareFusion, San Diego, California). The PImax was measured at residual volume as the subjects inhaled as hard and as quickly as possible. The PEmax was measured at total lung capacity as the subjects exhaled as hard and as quickly as possible. The PImax and PEmax were measured several times, and after 4 or 5 attempts, a plateau of values showed relatively little variability (± 10%).33
Cardiopulmonary Exercise Test
The cardiopulmonary exercise test is an incremental symptom-limited exercise test performed on an electronically braked cycle ergometer (Corival, Lode BV, Groningen, The Netherlands). The standard bicycle exercise ramp work load protocol was based on the method of Wasserman et al.34 The subjects were strongly encouraged to achieve their point of maximal exercise. Expired air was continuously analyzed using a cardiopulmonary diagnostic system (BreezeSuite 6.1, MGC Diagnostics, St. Paul, Minnesota) to assess physiologic responses to exercise. Oxygen uptake (V̇O2), carbon dioxide output, minute ventilation, breathing frequency, tidal volume, SpO2, end-tidal PCO2, electrocardiography, heart rate, and blood pressure were measured continuously during the exercise test. The Borg dyspnea scale was rated at rest and at peak exercise.
Pulmonary Rehabilitation
All subjects participated in 12-week (2 sessions/week) hospital-based out-patient PR. In each session, formal education, including breathing training, proper use of medications, and self-management skills, was given individually. After education, exercise training with a lower limb cycle ergometer was provided. Each training session was ∼40 min and was closely monitored by a rehabilitation therapist. Work rate, SpO2, heart rate, blood pressure, Borg dyspnea scale, and leg fatigue during exercise training were monitored.
Statistical Analysis
Baseline measurements and results after PR were expressed as mean ± SD. Paired t tests were used to compare measurements before and after PR. Statistical significance was set at P < .05. All statistical analyses were performed using SPSS 18.0 (SPSS, Chicago, Illinois). A post hoc power calculation was performed using the G*Power software to strengthen the substantial effect of PR on sleep quality (power > 80% to reject the null hypothesis with α = .05).
The sample size was estimated based on α level, β level, and effect size.35 The α level was set as .05, which indicates the probability of mistakenly rejecting the null hypothesis, and the β level was set as .20, which indicates the probability of mistakenly supporting the null hypothesis. The effect size was set as 0.2–0.5 using Cohen's d rules of one-sample study design, which indicates a small-to-medium effect of experimental intervention (PR) on the important outcomes (sleep quality). Finally, 34–52 subjects were need to participate in our study. The results indicated that the means ± SD of the PSQI for pretesting and posttesting were 9.41 ± 4.33 and 7.82 ± 3.90. The final effect size of PR on sleep quality was 0.41, which represented a small-to-medium effect size and indicated a substantial effect of PR on sleep quality in patients with COPD.
Results
Anthropometric and Spirometric Data
The baseline demographic and spirometric data of the subjects with COPD (Table 1) showed that the mean FEV1/FVC was 0.49 ± 0.13, and the mean FEV1 was 1.06 ± 0.49 L/min (49.7 ± 18.0% of predicted). Most subjects had moderate-to-severe COPD.
Baseline Characteristics of Subjects With COPD
Sleep Quality, Disease-Specific HRQOL, and Dyspnea Scores After PR
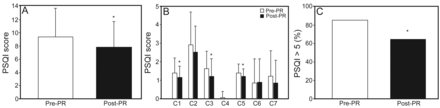
PR improved sleep quality (PSQI), HRQOL (SGRQ), and dyspnea scores (Fig. 1). After PR, the mean PSQI decreased from 9.41 ± 4.33 to 7.82 ± 3.90 (P < .001) (Fig. 1A). In the PSQI, sleep duration, sleep disturbance, and daytime dysfunction showed significant improvement after PR (Fig. 1B). Before PR, a majority of the subjects (85.3%) rated sleep as abnormal (PSQI > 5). This decreased to 64.7% after PR (Fig. 1C). There were also significant improvements in HRQOL and all domains of SGRQ after PR (symptoms, P = .003; activity, P = .03; impact, P = .02; total, P = .003) (Fig. 2). There was also significant improvement in exertional dyspnea after PR (P < .001) (Fig. 3).
Scoring and specific items on the Pittsburgh Sleep Quality Index (PSQI). A: After pulmonary rehabilitation (PR), the mean PSQI decreased from 9.41 ± 4.33 to 7.82 ± 3.90 (P < .05). B: Sleep duration, sleep disturbance, and daytime dysfunction showed significant improvement after PR. C: Before PR, the majority of patients (85.3%) rated sleep as abnormal (PSQI > 5). The number decreased to 64.7% after PR. Data are shown as mean ± SD. * Significant difference from pre-PR (P < .001). C1 = sleep quality; C2 = sleep latency; C3 = sleep duration; C4 = sleep efficiency; C5 = sleep disturbance; C6 = sleep medications; C7 = daytime dysfunction.
There were significant improvements in the St George Respiratory Questionnaire (SGRQ): total, symptoms, activity, and impact domains after pulmonary rehabilitation (PR). Data are shown as mean ± SD. * Significant difference from pre-PR (P < .001).
The Borg dyspnea scale at peak exercise showed significant improvement after pulmonary rehabilitation (PR). Data are shown as mean ± SD. * Significant difference from pre-PR (P < .001).
Physiologic Changes in Exercise Capacity, Pulmonary Function, and Respiratory Muscle Strength After PR
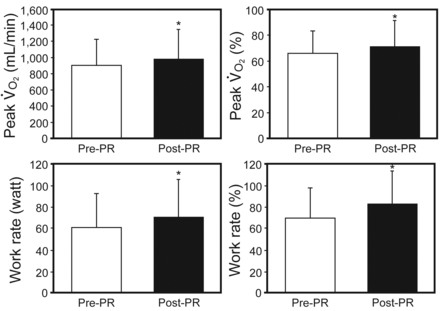
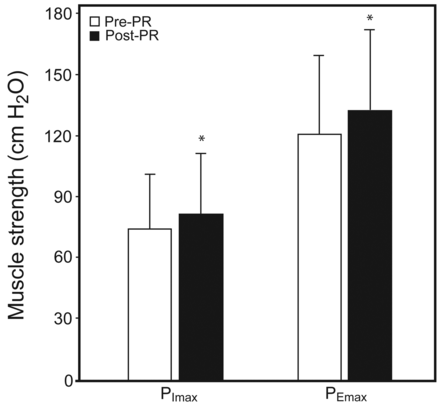
Post-PR changes in exercise capacity, pulmonary function, and respiratory muscle strength were demonstrated. After PR, there were significant improvements in exercise capacity, with increased maximal V̇O2 (mean increase 72.7 mL/min, P = .004) and work rate (mean increase 9.9 watts, P < .001) (Fig. 4). There were no significant changes in pulmonary function test results (FEV1, FVC, and FEV1/FVC) after PR (Fig. 5). Respiratory muscle strength significantly improved after PR ([PImax], P = .005; [PEmax], P = .004) (Fig. 6).
After pulmonary rehabilitation (PR), there were significant improvements in exercise capacity, with increased maximal oxygen uptake (V̇O2) (mean increase 72.7 mL/min, P = .004; 5.2%, P = .005) and work rate (mean increase 9.9 watts, P < .001; 12.7%, P < .001). Data are shown as mean ± SD. * Significant difference from pre-PR (P < .001).
After pulmonary rehabilitation (PR), there were no significant changes in pulmonary function test results. Data are shown as mean ± SD. * Significant difference from pre-PR (P < .001).
After pulmonary rehabilitation (PR), there were significant improvements in respiratory muscle strength. Data are shown as mean ± SD. * Significant difference from pre-PR (P < .001). PImax = maximum inspiratory pressure. PEmax = maximum expiratory pressure.
Discussion
This study has 2 major findings. First, the majority of subjects with COPD experienced poor sleep quality. Second, PR improved sleep quality with concurrent improvements of HRQOL, exercise capacity, and respiratory muscle strength. This study also demonstrated that exercise training improved sleep quality, increased sleep duration, and decreased sleep disturbance in subjects with COPD.
Studies on exercise training for improving sleep quality in COPD are limited. Nonetheless, some studies show that exercise training programs improve sleep health in other populations with different diseases and in middle-aged and older adults.36–38 Passos et al36 suggested that aerobic exercise may reduce presleep anxiety and improve sleep quality in patients with primary insomnia. King et al37 evaluated the effect of 4 months of exercise on sedentary individuals with reported sleep complaints and demonstrated that exercise programs improved their self-rated sleep quality. In the present study, an exercise program also had a positive effect on sleep quality in patients with COPD.
Poor sleep quality in patients with COPD is considered a result of multiple contributing factors, including disease-specific symptoms, concomitant medications, older age, underlying anxiety and depression, and presence of comorbidities.9 Respiratory disturbances during sleep have been associated with sleep-related oxygen desaturation, reduction in pulmonary function, and development of hypoventilation.39 Hypoventilation during sleep causes significant gas-exchange alteration, resulting in hypercapnia and hypoxia,9 which in turn lead to increased sleep arousals and sleep disruption.9 Moreover, there is a normal circadian change in airways leading to nocturnal bronchoconstriction with increased airway resistance.9 Intercostal muscle activity decreases during sleep, and this is particularly significant in patients with COPD because they are dependent on accessory muscle activity to maintain ventilation.9 The overall physiologic changes in COPD, including nocturnal hypoventilation, air-flow obstruction, hypoxia, hypercapnia, and use of accessory muscles of respiration, lead to poor sleep quality.40
Exercise is commonly believed to improve sleep quality.38 However, the exact mechanisms of how exercise training improves sleep quality in patients with COPD are unclear. Consideration of the mechanisms underlying the effect of exercise on sleep quality is beyond the scope of this study, but they are believed to consist of a complex set of activities, including physiological and psychological benefits.
Physiologically, PR improves muscle adaptations with increases in aerobic enzymes, further leading to improved respiratory muscle strength.23,41 This improvement is important in as much as it is associated with a reduction in dynamic hyperinflation23,24 and improves ventilation in patients with COPD. In addition, PR also leads to improvement of systemic inflammation.42 Pinho et al42 suggested that patients with COPD are characterized by increased systemic and pulmonary oxidative stress, and PR was associated with decreased oxidative stress. Exercise training has also been proposed to improve sleep quality by increasing energy consumption, endorphin secretion, and body temperature in a manner that facilitates sleep for body recuperation.38 Physical fitness is known to be associated with sleep quality,43 and greater improvements in fitness after exercise training are associated with better sleep outcomes.44
Psychologically, anxiety and depression can lead to poor sleep quality in patients with COPD.45 The prevalence of depression and anxiety is high in COPD.46 A meta-analysis of 6 randomized controlled trials concluded that PR is effective for the reduction of anxiety and depression.47 PR leads to better psychological status on the anxiety-depression scale in patients with COPD.20 It has been suggested that exercise training promotes sleep via its anxiolytic or antidepressant effects.48
There are multiple improvements resulting from PR in patients with COPD, including physiological and psychological benefits. Although the exact mechanisms of improvement of sleep quality after PR are not fully understood, exercise training could be an effective nonpharmacologic treatment for patients with COPD according to this study.
The present study has some limitations. First, the overlap of obstructive sleep apnea syndrome in patients with COPD may exacerbate poor sleep quality.49 In previous studies, the prevalence of overlap syndrome in COPD was 8.6–11%.50,51 Polysomnography was not performed in the current study, and the prevalence of organic sleep disorders in this study population could not be objectively determined. Therefore, it is hard to determine the influence of obstructive sleep apnea syndrome or other organic sleep disorders in this study. Second, nighttime oxygenation saturations were not measured. Nocturnal oxygen desaturation is an important factor contributing to sleep disturbances, but there were no data on nocturnal oxygenation in the study. Future studies on PR and sleep quality should incorporate this important measure. Third, only the short-term effect of PR on sleep quality was evaluated. It is necessary to perform long-term studies to evaluate the duration of improvement in sleep quality after 3 months of PR.
Conclusions
Patients with COPD often have poor sleep quality. PR may provide significant improvement in sleep quality, with concurrent improvement in HRQOL and exercise capacity. As such, PR can be recommended as an effective nonpharmacologic treatment to improve sleep quality in patients with COPD, and it should be part of their clinical management.
Footnotes
- Correspondence: Dr Yao-Kuang Wu, Division of Pulmonary Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, No. 289, Jianguo Road, New Taipei City 23142, Taiwan. E-mail: drbfci{at}yahoo.com.tw.
The authors have disclosed no conflicts of interest.
- Copyright © 2014 by Daedalus Enterprises