Abstract
BACKGROUND: We tested the association between pulmonary dead-space fraction (ratio of dead space to tidal volume [VD/VT]) and mortality in subjects with ARDS (Berlin definition, PaO2/FIO2 ≤ 300 mm Hg; PEEP ≥ 5 cm H2O) enrolled into a clinical trial incorporating lung-protective ventilation.
METHODS: We conducted a prospective, multi-center study at medical-surgical ICUs in the United States. A total of 126 ALI subjects with acute lung injury were enrolled into a phase 3 randomized, placebo-controlled study of aerosolized albuterol. VD/VT and pulmonary mechanics were measured within 4 h of enrollment and repeated daily on study days 1 and 2 in subjects requiring arterial blood gases for clinical management.
RESULTS: At baseline, non-survivors had a trend toward higher VD/VT compared with survivors (0.62 ± 0.11 vs 0.56 ± 0.11, respectively, P = .08). Differences in VD/VT between non-survivors and survivors became significant on study days 1 (0.64 ± 0.12 vs 0.55 ± 0.11, respectively, P = .01) and 2 (0.67 ± 0.12 vs 0.56 ± 0.11, respectively, P = .004). Likewise, the association between VD/VT and mortality was significant on study day 1 (odds ratio per 0.10 change in VD/VT [95% CI]: 6.84 [1.62–28.84] P = .01; and study day 2: 4.90 [1.28–18.73] P = .02) after adjusting for VD/VT, PaO2/FIO2, oxygenation index, vasopressor use, and the primary risk for ARDS. Using a Cox proportional hazard model, VD/VT was associated with a trend toward higher mortality (HR = 4.37 [CI 0.99–19.32], P = .052) that became significant when the analysis was adjusted for daily oxygenation index (HR = 1.74 [95% CI 1.12–3.35] P = .04).
CONCLUSIONS: Markedly elevated VD/VT (≥ 0.60) in early ARDS is associated with higher mortality. Measuring VD/VT may be useful in identifying ARDS patients at increased risk of death who are enrolled into a therapeutic trial.
- acute lung injury
- acute respiratory distress syndrome
- mechanical ventilation
- physiologic dead-space fraction
- single-breath test for carbon dioxide
Introduction
Physiologic dead-space fraction (ratio of dead space to tidal volume [VD/VT]) is the portion of tidal volume that does not participate in gas exchange and therefore consists of expired gas without carbon dioxide. Historically, elevated VD/VT in patients with ARDS was thought to be a late-occurring phenomenon associated with the fibroproliferative stage of injury.1 However, newer evidence indicates that VD/VT is markedly elevated within 24 h of ARDS onset and is significantly elevated in non-survivors.2–8 Moreover, sustained elevation of VD/VT in ARDS has been associated with higher mortality.5,6
A pulmonary-specific physiologic variable such as VD/VT, which is strongly associated with mortality, could be useful in assessing the efficacy of new therapies for ARDS in prospective clinical trials. Prior studies examining the prognostic value of VD/VT have had limitations that prevent the generalizability of their results to subjects eligible for therapeutic clinical trials, including the fact that subjects were studied at only 1 or 2 hospitals.2–8 Older studies,2,6 in which subjects were managed with traditional higher VT ventilation, did not always include subjects with less severe oxygenation defects (ie, these studies focused only on subjects with moderate or severe ARDS by the current Berlin definition),9 and included subjects who had risk factors for mortality that would have excluded them from enrollment in a therapeutic clinical trial.
More recent, small, prospective studies3–5,10 enrolled subjects spanning the spectrum of ARDS from mild to severe, who were managed with lung-protective ventilation. In these studies, early elevation in VD/VT was associated with increased mortality. However, the results of these studies differed in whether abnormalities in VD/VT differentiated subjects with mild versus severe oxygenation defects. In one study,10 elevated VD/VT alone did not predict mortality unless it was associated with elevated plasma markers for endothelial damage.
To our knowledge, only one study has incorporated VD/VT into a therapeutic clinical trial to assess mortality risk. In a phase 2 randomized, controlled, multi-centered study of 75 subjects with ARDS,11 VD/VT was used to assess the physiologic effects of recombinant activated protein C on pulmonary function. In that trial, which managed subjects with the ARDS Network lung-protective ventilation protocol,12 there was a significant decline in VD/VT among subjects who received recombinant activated protein C.11 However, mortality was exceptionally low (13%), and the association between VD/VT and mortality was not addressed specifically.
The current study was designed to determine whether VD/VT in patients with ARDS (PaO2/FIO2 ≤ 300 mm Hg)9 is associated with mortality in the context of a large clinical trial using lung-protective ventilation. We prospectively studied subjects enrolled into a multi-center, phase 3, randomized-controlled trial of the National Heart, Lung and Blood Institutes' ARDS Network.13 Our primary objective was to determine whether elevated VD/VT early in the clinical course was associated with mortality.
QUICK LOOK
Current knowledge
Elevated physiologic dead-space fraction (ratio of dead space to tidal volume [VD/VT]) is a marker of the severity of lung injury in ARDS. Recent studies suggest VD/VT is markedly elevated in the first 24 h after ARDS onset and that sustained elevation of VD/VT is associated with an increased mortality.
What this paper contributes to our knowledge
In patients with ARDS as defined by the Berlin definition (PaO2/FIO2 ≤ 300 and PEEP ≥ 5 cm H2O), a VD/VT ≥ 0.60 was associated with a higher mortality. The role of routine monitoring of VD/VT to predict outcome or guide therapy remains to be determined.
Methods
Subjects 18 y or older were co-enrolled into this observational sub-study of VD/VT within 48 h of meeting the American-European Consensus Conference criteria for acute lung injury or ARDS.14 Specific inclusion and exclusion criteria have been previously published.13 To qualify for the study, subjects had to meet all 3 American-European Consensus Conference criteria (PaO2/FIO2 ≤ 300, bilateral infiltrates on chest radiograph during invasive mechanical ventilation, and the absence of evidence of elevated left atrial pressures) within the same 24-h period. In addition, enrollment, randomization, and initial protocol-directed therapies had to be initiated within 48 h of meeting acute lung injury or ARDS criteria. Of the 22 specific exclusion criteria, those most relevant to the dead-space sub-study were severe chronic respiratory disease, which was defined as chronic hypercapnia with PaCO2 > 45 mm Hg, chronic hypoxemia with PaO2 < 55 mm Hg on room air, secondary polycythemia, severe pulmonary hypertension with mean PAP > 40 mm Hg, or ventilator dependence; diffuse alveolar hemorrhage from vasculitis, severe morbid obesity, and moribund condition (ie, not expected to survive 24 h). A complete list of criteria can be found online at http://www.clinicaltrials.gov/ct2/show/NCT00434993.13
Subjects were enrolled between August 6, 2006 and July 7, 2008 at 24 hospitals of the National Heart, Lung, and Blood Institute ARDS Network (ClinicalTrials.gov identifier NCT00434993, Appendix). The VD/VT sub-study was approved by the data safety monitoring board for the parent clinical trial,13 as well as by the institutional review board of each participating hospital. Written informed consent was obtained from subjects or their surrogates at the time of enrollment into the clinical treatment trial.
Measurements
Measurements of VD/VT were performed within 4 h of enrollment and repeated daily on study days 1 and 2 if arterial blood gas measurements were indicated for clinical management. An automated volumetric capnography monitor was used (NICO, Philips Respironics Healthcare, Wallingford, Connecticut) that had been previously validated in patients with ARDS.15 Mean expired carbon dioxide measurements with the NICO monitor coincided with arterial blood gas procurement and a ventilator systems check.
Assessments were made only when subjects were managed with a ventilator mode providing full support (ie, volume, pressure, or dual-mode assist/control ventilation), so that inspiratory time and VT were likely to be relatively stable, and measurements of respiratory-system compliance could be made. Subjects were studied in the semi-recumbent position, in the absence of nursing care activities and when they were observed to be calm and synchronous with the ventilator.
In addition to VD/VT, the mean expired carbon dioxide partial pressure, volume of carbon dioxide excretion per minute, and expired VT were recorded from the NICO monitor. We also recorded arterial blood gas values and standard ventilator data such as ventilator mode, plateau pressure, PEEP, mean airway pressure, FIO2, and total breathing frequency.
VD/VT was calculated by the monitor using the Enghoff modification of the Bohr equation as the difference between arterial and mean expired carbon dioxide partial pressure divided by the arterial carbon dioxide partial pressure16: VD/VT = (PaCO2 − PĒCO2)/PaCO2. Minute ventilation was calculated as the product of expired VT and total breathing frequency. Respiratory-system compliance was calculated as VT divided by the end-inspiratory plateau pressure minus PEEP. Oxygenation index (OI) was calculated as the product of mean airway pressure and the percent of inspired oxygen divided by the partial pressure of arterial oxygen.17
Each participating site received formal training on the use of the NICO monitor provided by clinical research specialists from Philips Respironics Healthcare. The training material and presentation was designed by Philips Respironics Healthcare and ARDS Network investigators, and was based on the same training program developed for a previous multi-center clinical trial.11
Because numerous clinicians across multiple research centers were making measurements of VD/VT, two simple quality control measures were used to verify data prior to the analysis. The first was to confirm that dead-space measurements were made on a full support mode of ventilation. Second, to lessen the possibility of inadvertent transcription error, the recorded VD/VT was verified by independent calculation using the Enghoff-Bohr equation and the corresponding recorded values for PaCO2 and PĒCO2. Although recording errors could have occurred in either direction, a pre hoc decision was made that both calculations had to be in agreement in order for data to be included in the analysis.
Death prior to hospital discharge (or hospital day 90) was the primary outcome variable in this study. Subjects were followed until death or discharge from the hospital.
Statistical Analysis
Continuous variables were expressed as mean ± SD or median with interquartile range, and were compared using Student t test or the Wilcoxon rank-sum test, where appropriate. Categorical variables were reported as percentages and compared using chi-square tests or Fisher exact tests where appropriate. Multivariate logistic regression models were used to test the association of VD/VT with mortality. A pre hoc decision was made to adjust the analyses for ARDS etiology, OI, ratio of PaO2 to FIO2 (PaO2/FIO2), and for the presence of shock (defined as the use of vasopressors except for dopamine at a dose of ≤ 5 mcg/kg/min) as a measure of severity of illness. Although the Acute Physiology and Chronic Health Evaluation (APACHE) III score was calculated, it was not used in the modeling for practical reasons as the score is not available in clinical practice, whereas information regarding vasopressor use is and is associated with higher mortality.18 However, the primary etiology causing ARDS was categorized as pneumonia, sepsis, aspiration, trauma, and other, and then entered into the model as dummy variables. The etiology of ARDS was determined by study investigators through review of the medical record and recorded for all study subjects. The odds ratio (OR) for death was calculated per 0.10 increases in VD/VT.
Two additional tests were done to assess the potential impact of VD/VT on mortality over time. First, analysis of covariance was used to assess differences in VD/VT between non-survivors versus survivors at day 2, adjusting for baseline VD/VT. Second, Cox proportional-hazards models were used to test the association between VD/VT and mortality in the subgroup of subjects who had complete data over the first 3 days. For this purpose, we constructed 3 models. Model 1 was unadjusted and only included VD/VT measured on a daily basis over the first 3 days as a time-varying covariate. Model 2 included daily VD/VT and baseline OI as the covariates. Model 3 included daily VD/VT and daily OI as time-varying covariates. We selected OI as a covariate in these models because of prior studies showing a strong association with mortality.19
All results were considered to be statistically significant at two-tailed P < .05. Stata 12.0 (Stata Corp, College Station, Texas) computer software was used for statistical analysis.
Results
When the primary clinical trial was stopped, a total of 354 dead-space measurements had been made in 126 subjects. The quality control assessment revealed that 308 measurements (87%) in 115 subjects (90%) were done on an approved full-support mode of ventilation, and also passed the secondary data-validity check. For these 115 subjects, the 60-day mortality was 19%. Sixteen subjects did not have baseline measurements made on the day of study enrollment. Therefore, 99 subjects had dead-space measurements made at baseline. Dead-space measurements were made in 84 subjects on study day 1, and in 56 subjects on study day 2 (Fig. 1). The primary etiology for lung injury was pneumonia followed by sepsis, aspiration, and trauma. Non-survivors were older, and at baseline had both significantly higher APACHE III scores and higher vasopressor use (Table 1). Over the duration of the study, pulmonary gas exchange dysfunction was characterized by elevated VD/VT and diminished PaO2/FIO2, as well as markedly decreased respiratory-system compliance (Table 2).
Flow chart.
Subjects Characteristics
Pulmonary Dead-Space Fraction and Other Respiratory Variables
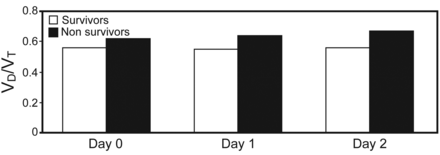
When analyzed by outcome, there was a trend toward higher baseline VD/VT in non-survivors compared with survivors (0.62 ± 0.11 vs 0.56 ± 0.11, respectively, P = .08). However, VD/VT was significantly higher among non-survivors on study day 1 (0.64 ± 0.12 vs 0.55 ± 0.11, respectively, P = .01) and day 2 (0.67 ± 0.12 vs 0.56 ± 0.11, respectively, P = .004) (Fig. 2). Likewise, the OR for death in the unadjusted logistic regression model approached statistical significance on the day of study enrollment (OR = 1.59 [95% CI 0.94–2.72] for every 0.10 increase in VD/VT, P = .08); thereafter, the association between VD/VT and mortality was stronger, becoming statistically significant on study days 1 (OR = 1.94 [95% CI 1.16–3.27], P = .01) and day 2 (OR = 2.50 [95% CI 1.26–4.97], P = .009) (Table 3).
Dead-space fraction (VD/VT) and outcome by study day.
Mortality as a Function of Dead-Space Fraction by Unadjusted and Adjusted Analyses
Adjusting the analysis for ARDS etiology, PaO2/FIO2, OI, and baseline vasopressor use produced a modest increase in the baseline OR for VD/VT: (OR = 1.73 [0.82–3.63], P = .09). However, the strength of association between VD/VT and mortality in the adjusted model increased markedly on study day 1 (OR = 6.84 [1.62–28.84], P = .01) and study day 2 (OR = 4.90 [1.28–18.73], P = .02) (Table 3). In contrast, only baseline PaO2/FIO2 and vasopressor use were significantly associated with mortality in the adjusted model.
Next, we used analysis of covariance to test whether differences in VD/VT between non-survivors and survivors at day 2 remained significant after controlling for baseline VD/VT. Indeed, day 2 VD/VT remained significantly associated with mortality in this model (P = .03). In an alternate analysis, we used Cox proportional hazard modeling with VD/VT as a time-varying covariate, VD/VT was associated with a trend toward higher mortality (HR = 4.37 [CI 0.99–19.32], P = .052) per 0.10 VD/VT increase. This difference became significant when the analysis was adjusted for daily OI (HR = 5.69 [95% CI 1.13–28.62], P = .04). The HR of mortality for VD/VT after adjusting for baseline OI was 4.28 (95% CI 0.86–21.39, P = .08).
Finally, because this study was done within a larger randomized, placebo-controlled clinical trial assessing the efficacy of aerosolized albuterol,13 analysis of covariance was used to assess the potential influence of albuterol on VD/VT at day 2, controlling for baseline level. Aerosolized albuterol therapy did not affect VD/VT (P = .84).
Discussion
The primary objective of this study was to assess whether VD/VT was associated with mortality in subjects with ARDS enrolled into a prospective clinical trial, and whether subsequent measurements were equally useful compared with those made at study entry. On the day of study enrollment, there was a trend toward higher VD/VT in non-survivors. On both study days 1 and 2, VD/VT was significantly higher in non-survivors. These results suggest the possibility that VD/VT measured on the first few days following enrollment might be an even better tool for assessing mortality risk. Of note, the association between VD/VT and mortality was independent of the degree of oxygenation impairment, a finding that is consistent with other studies.2,5 Moreover, the adjusted analyses demonstrated that, in contrast to measures of oxygenation, the association between VD/VT and mortality was stronger and remained significant over the first 3 study days.
The difference in VD/VT between non-survivors and survivors of ARDS in this study is similar to that reported by several other investigators. In 3 prior studies,4–6 the average VD/VT on study enrollment was 0.61–0.62 in non-survivors and 0.53–0.54 in survivors. In another study3 of patients with mild ARDS, the difference was 0.55 and 0.48 for non-survivors and survivors, respectively. Among studies that made repeated measurements over several days, the gap in VD/VT between non-survivors and survivors was sustained or increased.3,5,6 In these studies, the initial difference in VD/VT between non-survivors and survivors was 0.06–0.08, and increased to 0.1 or greater over the disease course.3,5,6 These previous findings are similar to our results, in which the initial difference in mean VD/VT between non-survivors and survivors was 0.06 and subsequently increased to 0.09–0.11. A plausible explanation for the consistent results across studies is that pathophysiologic changes in ARDS (as manifested by VD/VT) may be more severe in non-survivors and appear to progress, whereas, among survivors, the pathophysiologic changes are less severe and/or self-limiting.
In ARDS, PEEP has a variable effect upon VD/VT: alveolar recruitment decreases dead space, whereas alveolar over-distention increases it.20 It is difficult to predict the impact of PEEP because both phenomena can occur simultaneously. In our study, PEEP and FIO2 were adjusted according to the ARDS Network low VT protocol based upon a target PaO2 range of 55–80 mm Hg, rather than optimizing either pulmonary oxygenation or pulmonary mechanics. Therefore, it is uncertain how protocol-directed changes in PEEP may have influenced VD/VT, particularly in subjects whose pulmonary function was deteriorating. However, measurements obtained during the first 3 days of study showed no difference between non-survivors and survivors in PEEP, VT or plateau pressure (surrogates of potential pulmonary over-distention). These findings suggest that there was no systematic difference in how these variables were adjusted between non-survivors and survivors that may have influenced dead-space measurements.
The major limitation of this study was that a sufficient number of subjects could not be enrolled to adequately test whether the association between VD/VT and mortality was different depending on the initial severity of hypoxemia (ie, in subgroups as per the Berlin definition according to a PaO2/FIO2 ratio < 100 mm Hg, 100–200 mm Hg, or > 200 mm Hg).9 Another potential limitation stems from the fact that daily measurements occurred only in subjects who had arterial blood gas analysis ordered for clinical management. Therefore, a potential bias is that our study sample may have represented more subjects, who by clinical presentation may have been judged to be deteriorating by clinicians caring for them, or at least more tenuous than those who did not have arterial blood gas measurements. Regardless these would encompass the very subjects in whom the predictive potential of dead-space measurements would be most useful.
Another relevant issue has been the search for a readily available surrogate of VD/VT that eliminates the need for expired gas monitoring. This has been particularly important to those involved with population-based outcome studies of ARDS. Interest in the relationship of CO2 excretion to mortality is stymied by the fact that dead-space measurements are not yet standard clinical practice. For example, the ARDS Definition Task Force9 attempted to use corrected minute ventilation (ie, [PaCO2 × minute ventilation]/40)21 as a potential surrogate for dead space in defining those with severe lung injury. However, this surrogate was not used in the final definition because of a lack of evidence for predictive validity.9
Others have reported that estimated VD/VT (by calculating carbon dioxide production from the Harris-Benedict equation, in conjunction with a modified alveolar air equation to derive mean PĒCO2) was useful for predicting mortality in ARDS.22 This encouraging result seemingly obviates direct measurement of expired CO2 in clinical practice. However, these findings should be interpreted with caution because of issues concerning validation methodology23,24 In addition, there is clinical evidence that this method significantly underestimates actual dead space; therefore, it may not be an ideally suited tool for evaluating the true impact of impaired CO2 excretion on outcomes in ARDS.25
The uncertainty surrounding estimated versus measured VD/VT is based in part upon findings that equations used to predict metabolism agree poorly with measured energy expenditure in critically-ill, mechanically-ventilated patients.26 In addition, the measured volume of CO2 excreted by the lungs (which determines mean PĒCO2) is unlikely to reflect CO2 production in the presence of severe ventilation:perfusion mismatching, intrapulmonary shunting, and shock.27 This disparity between production and excretion during critical illness reflects the body's considerably capacity to store CO2 (estimated to reach 20 L, or 11.6 mL/kg per 1 mm Hg change in PaCO2); the dynamics of which are partly determined by muscle perfusion.28,29 In fact, even under normal physiologic conditions, a true CO2 steady state is considered rare.29 Given these uncertainties, and until better methods of accurately estimating PeCO2 have been firmly established, VD/VT should be determined in subjects with ARDS using direct measurements of expired CO2.
Conclusions
The results of this study demonstrate the practicality and utility of measuring VD/VT in subjects with ARDS enrolled in a clinical trial. In addition, this relatively large multi-center observational study confirms the results of previous smaller, single-center studies,2–6 specifically that early and sustained elevations in VD/VT are associated with higher mortality in patients with ARDS. Therefore, measurement of VD/VT appears to provide important information that may be useful in therapeutic clinical trials.
Acknowledgments
We would particularly like to thank Ivan Bustamante RRT and James Bement RRT for their extraordinary efforts in providing training to both Network personnel and staff at participating hospitals. We are indebted to ICU personnel, especially respiratory therapists and nurses, and to our subjects and their families, who supported this trial.
National Heart Lung and Blood Institute Acute Respiratory Distress Syndrome Network Investigators (site principal investigators are indicated with an asterisk)
Baystate Medical Center, Springfield, Massachusetts: JS Steingrub,* M Tidswell, L DeSouza, C Kardos, L Kozikowski, K Kozikowski.
Johns Hopkins Hospital, Baltimore, Maryland: RG Brower,* HE Fessler, DN Hager, PA Mendez-Tellez, K Oakjones, DM Needham.
Johns Hopkins Bayview Medical Center, Baltimore, Maryland: J Sevransky, A Workneh, S Han, S Murray.
University of Maryland, Baltimore, Maryland: C Shanholtz, G Netzer, P Rock, A Sampaio, J Titus, T Harrington.
Washington Hospital Center, Washington, DC: D Herr, B Lee, N Bolouri, GR Khorjekar.
Cleveland Clinic Foundation, Cleveland, Ohio: HP Wiedemann,* RW Ashton, DA Culver, T Frederick, JJ Komara, JA Guzman, AJ Reddy.
University Hospitals of Cleveland, Cleveland, Ohio: R Hejal, M Andrews, D Haney.
MetroHealth Medical Center, Cleveland, Ohio: AF Connors, S Lasalvia, JD Thornton, EL Warren.
University of Colorado Health Science Center, Aurora, Colorado: M Moss,* AB Benson, EL Burnham, BJ Clark, L Gray, C Higgins, BJ Maloney, M Mealer.
Denver Health Medical Center, Denver, Colorado: I Douglas, K Overdier, K Thompson, R Wolken.
University of North Carolina, Chapel Hill, North Carolina: SS Carson, L Chang, J Lanier.
Wake Forest University, Winstom-Salem, North Carolina: RD Hite,* PE Morris, A Howard, A Harvey, K Bender.
Moses Cone Memorial Hospital, Greensboro, North Carolina: PE Wright, C Carter-Cole, S Gross, J McLean, A Overton.
University of Virginia, Charlottesville, Virginia: JD Truwit, K Enfield, M Marshall, CG Irvin.
LDS Hospital, Salt Lake City, Utah: TP Clemmer, LK Weaver, J Gleed.
Cottonwood Hospital, Murray, Utah: M Zenger, J Krueger.
Intermountain Medical Center, Murray, Utah: AH Morris,* A Ahmed, A Austin, NC Dean, CK Grissom, E Hirshberg, N Kumar, RR Miller, L Napoli, JF Orme, S Pandita, G Schreiber, L Struck, F Thomas, GE Thomsen.
McKay-Dee Hospital, Ogden, Utah: C Lawton, F Leung, P Kim, T Fujii, J Baughman, B Kerwin, D Hanselman, J d'Hulst.
Utah Valley Regional Medical Center, Provo, Utah: KM Sundar, W Alward, T Hill, EJ Campbell, KA Ludwig, DB Nielsen, MJ Pearce.
University of California San Francisco, San Francisco, California: MA Matthay,* CS Calfee, BM Daniel, M Eisner, O Garcia, ER Johnson, K Kordesch, KD Liu, H Zhou.
University of California San Francisco, Fresno, California: MW Peterson, J Blaauw.
University of California Davis, Davis, California: TE Albertson, E Vlastelin.
San Francisco General Hospital, San Francisco, California: JV Diaz, ER Johnson, RH Kallet.
Mayo Foundation, Rochester, Minnesota: RD Hubmayr,* DR Brown, O Gajic, R Hinds, SR Holets, DJ Kor, M Passe.
Louisiana State University, Shreveport, Louisiana: BP deBoisblanc,* P Lauto, C Romaine, G Meyaski, JP Hunt, A Marr.
Louisiana State University Health Sciences Center, Earl K Long Medical Center; Baton Rouge General Medical Center Mid-City; and Baton Rouge General Medical Center Bluebonnet, Baton Rouge, Louisiana: S Brierre, C LeBlanc, T Jagneaux.
Alton-Ochsner Clinic Foundation, New Orleans, Louisiana: DE Taylor. S Jain, L Seoane.
Tulane University, New Orleans, Louisiana: F Simeone, J Fearon, J Duchesne.
Clinical Coordinating Center (Massachusetts General Hospital and Harvard Medical School), Boston, Massachusetts: DA Schoenfeld,* M Aquino, D Dorer, M Guha, E Hammond, N Lavery, P Lazar, I Molina, R Morse, CF Oldmixon, B Rawal, NJ Ringwood, A Shui, E Smoot, BT Thompson.
National Heart, Lung and Blood Institute, Bethesda, Maryland: AL Harabin, S Bredow, MA Waclawiw, GG Weinmann.
Data and Safety Monitoring Board: RG Spragg (chair), AS Slutsky, MM Levy, BP Markovitz, E Petkova, C Weijer, DF Willson.
Protocol Review Committee: JI Sznajder (chair), M Begg, E Israel, J Lewis, PE Parsons
Footnotes
- Correspondence: Richard H Kallet MSc RRT FAARC, Department of Anesthesia, University of California San Francisco at San Francisco General Hospital, NH:GA-2, 1001 Potrero Avenue, San Francisco, CA 94110.
This study was supported by National Heart, Lung and Blood Institute contracts NO1-HR-56165–56713, and by material support (the loan of monitors, disposables and training) from Philips Respironics. RH Kallet and MA Matthay have received similar past support from Philips Respironics for other clinical trials. The other authors have disclosed no conflicts of interest.
- Copyright © 2014 by Daedalus Enterprises