Abstract
BACKGROUND: Transcutaneous carbon dioxide (PtcCO2) monitoring is being used increasingly to assess acute respiratory failure. However, there are conflicting findings concerning its reliability when evaluating patients with high levels of PaCO2. Our study evaluates the accuracy of this method in subjects with respiratory failure according to the severity of hypercapnia.
METHODS: We included subjects with respiratory failure, admitted to a respiratory intermediate care unit, who required arterial blood gas analysis. Simultaneously, PtcCO2 was measured using a digital monitor. Relations between PaCO2 and PtcCO2 were assessed by the Pearson correlation coefficient. Bland-Altman analysis was used to test data dispersion, and an analysis of variance test was used to compare the differences between PaCO2 and the corresponding PtcCO2 at different levels (level 1, <50 mm Hg; level 2, 50–60 mm Hg; level 3, >60 mm Hg).
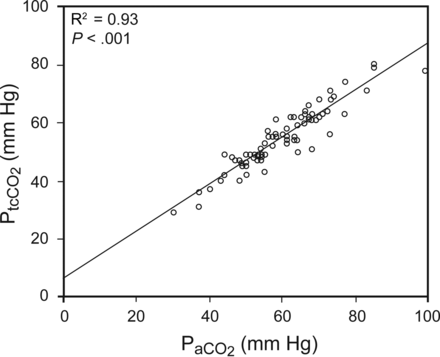
RESULTS: Eighty-one subjects were analyzed. The main diagnosis was COPD exacerbation (45%). PtcCO2 correlated well with PaCO2 (r2 = 0.93, P < .001). Bland-Altman analysis showed a mean PaCO2 − PtcCO2 difference of 4.9 ± 4.4 with 95% limits of agreement ranging from −3.6 to 13.4. The difference between variables increased in line with PaCO2 severity: level 1, 1.7 ± 3.2 mm Hg; level 2, 3.7 ± 2.8; level 3, 6.8 ± 4.7 (analysis of variance, P < .001).
CONCLUSIONS: Our study showed an acceptable agreement of PtcCO2 monitoring with arterial blood gas analysis. However, we should consider that PtcCO2 underestimates PaCO2 levels, and its accuracy depends on the level of hypercapnia, so this method would not be suitable for acute patients with severe hypercapnia.
- hypercapnia
- noninvasive ventilation
- respiratory care units
- respiratory insufficiency
- transcutaneous blood gas monitoring
- transcutaneous carbon dioxide partial pressure determination
- transcutaneous capnometry
Introduction
Transcutaneous carbon dioxide (PtcCO2) monitoring is an increasingly popular noninvasive method for assessing alveolar ventilation. This procedure has the advantage over arterial blood samples of avoiding pain and providing continuous monitoring.
Several studies have reported a good agreement between PtcCO2 and PaCO2 in different subject populations, such as: pediatric critical care,1 sleep laboratories,2 out-patients,3 during major surgery,4 in emergency departments,5 and ICUs.6
The accuracy of PtcCO2 has also been studied in acute respiratory failure settings with a variety of results, which range from a very good agreement to unacceptable results for clinical practice.6–12 This controversy could be explained by the heterogeneity of diagnosis and by the fact that the number of subjects with severe ventilatory abnormalities is very different in these studies.
However, when we look at the studies that have evaluated subjects with high levels of PaCO2, conflicting findings are continuously observed. Some authors suggest that at high PaCO2 values, larger differences between PtcCO2 and PaCO2 are observed.9,13 In contrast, other studies6–8 consider that the agreement between the 2 methods is independent of the level of PaCO2. These differences are especially relevant in situations of acute respiratory failure, when the level of hypercapnia may condition therapeutic decisions, such as the need for mechanical ventilation. The aim of our study was to assess the accuracy of PtcCO2 in subjects with acute respiratory failure according to the severity of hypercapnia.
QUICK LOOK
Current knowledge
Transcutaneous carbon dioxide (PtcCO2) monitoring is an increasingly popular noninvasive method for assessing acute respiratory failure. This procedure has the advantage over arterial blood samples of avoiding pain and providing continuous monitoring. However, there are conflicting findings concerning its reliability when evaluating patients with high levels of PaCO2.
What this paper contributes to our knowledge
In subjects with acute respiratory failure, a portable PtcCO2 device provided an acceptable assessment of PaCO2 when compared with the accepted standard measurement of PaCO2. However, PtcCO2 underestimated PaCO2 levels, and its accuracy depended on the level of hypercapnia. PtcCO2 was less reliable in subjects with severe hypercapnia.
Methods
Study Design
This was a prospective observational study. Written informed consent was not considered necessary for the study, given its non-interventional nature. The data of the subjects were anonymized for the purposes of analysis. Confidential information regarding subjects was protected according to Spanish regulations. The study was approved by the Clinical Research Ethics Committee of Bellvitge University Hospital.
Study Setting and Population
The study was conducted in a respiratory intermediate care unit at the Bellvitge University Hospital, a teaching hospital with 600 beds, between June 2012 and December 2013. The annual new patient attendance at this respiratory intermediate care unit is approximately 250. Patients with respiratory failure (acute or acute-on-chronic) who required arterial blood gas analysis as a part of their care were eligible for inclusion.
Study Protocol, Measurements, and Data Collection
Eligible subjects were identified and enrolled by one of the senior respiratory intermediate care unit physicians trained in the use of the PtcCO2 monitor. PtcCO2 was measured using a digital monitor (SenTec, Therwill, Switzerland). After automatic calibration, carried out according to the manufacturer's recommendations, a probe was placed on the subject's upper chest using a drop of conductive solution. The sensor was heated to 42°C over 5 min, inducing local vasodilatation to increase skin permeability to CO2. A minimum of 20 min was allowed for stabilization, and an arterial blood gas sample was obtained from the radial artery. The PtcCO2 reading on the monitor at the moment of the withdrawal of the needle was recorded. Arterial blood gases were analyzed immediately using an ABL800 FLEX gas analyzer (Radiometer, Copenhagen, Denmark) and expressed in mm Hg.
The data collected included subject demographics, body mass index, cause of respiratory failure, vital signs, use of vasoactive drugs, oxygen (FIO2) or use of noninvasive ventilation, arterial blood gas analysis, and PtcCO2 measurements.
Data Analysis
Descriptive data are presented as mean ± SD and as n (%). The Pearson correlation coefficient was used to evaluate the linear relationship between the paired arterial and transcutaneous carbon dioxide measurements. Agreement between these 2 variables was estimated using the Bland-Altman method. Bias (the mean difference between PaCO2 and PtcCO2) and 95% limits of agreement were calculated. With the purpose of analyzing the behavior of the PtcCO2 in relation to hypercapnia, an analysis of variance test was performed to compare the bias between paired PaCO2-PtcCO2 measurements at different levels of PaCO2 (level 1, <50 mm Hg; level 2, 50–60 mm Hg; level 3, >60 mm Hg). Values of P < .05 were considered statistically significant. Statistical tests were run using SPSS 16 for Windows (SPSS, Chicago, Illinois).
Results
Ninety-five subjects were enrolled in the study. Eleven measurements were excluded because venous blood gases were drawn, and technical problems with the monitor led to the exclusion of 3 subjects. A total of 81 subjects were analyzed with paired measurements for arterial and transcutaneous carbon dioxide tension recorded for each one.
The clinical characteristics of the subjects included are presented in Table 1. The mean age was 66 ±11 y. COPD exacerbation (34 subjects or 45%) was the main final diagnosis, followed by obesity hypoventilation syndrome (22 subjects or 22%). At the time of arterial blood sampling, 72% of the subjects were receiving oxygen, and 38% were receiving noninvasive ventilation. None of the subjects studied were in shock or hypothermic, and only 2 required inotropic support. Mean PaCO2 was 59.8 ± 11.9 mm Hg (range 30–99 mm Hg), and mean PtcCO2 was 54.9 ± 10.4 mm Hg (range 29–80 mm Hg). In relation to PaCO2 level, subjects were distributed as follows: level 1 (<50 mm Hg), 13 (16%); level 2 (50–60 mm Hg), 29 (36%); level 3 (>60 mm Hg), 39 (48%). The differences between paired PaCO2-PtcCO2 measurements at different levels of PCO2 are provided in Table 2.
Characteristics of Study Subjects (N = 81)
Agreement Between PaCO2 and PtcCO2 at Different Levels of PaCO2
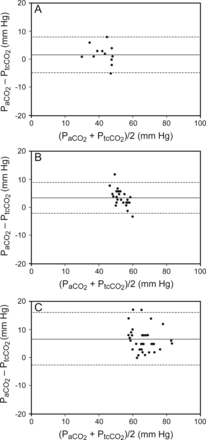
There was a high correlation between PaCO2 and PtcCO2, as shown in Figure 1; R2 was 0.93 (P < .001). The Bland-Altman plot of all the subjects included in the study is presented in Figure 2. The mean bias between the 2 methods of measurement was 4.9, and limits of agreement (bias ± 1.96 SD) ranged from −3.6 to 13.4. We also calculated the bias and the limits of agreement between the parameters for each group separately (Fig. 3). In the group of more severe hypercapnic subjects with PaCO2 > 60 mm Hg, the Bland-Altman test showed a mean bias of 6.7 and limits of agreement from −2.5 to 16.1.
Linear correlation between transcutaneous CO2 (PtcCO2) and arterial CO2 (PaCO2).
Bland-Altman analysis between paired measurements of arterial PCO2 (PaCO2) and transcutaneous CO2 (PtcCO2). The solid line represents the mean difference between the 2 methods, and the dotted lines denote the limits of agreement (±1.96 SD).
Bland-Altman analysis between paired measurements of arterial PCO2 (PaCO2) and transcutaneous CO2 (PtcCO2) for each group of subjects: level 1 PaCO2 <50 mm Hg (A); level 2 PaCO2 50–60 mm Hg (B); level 3 PaCO2 >60 mm Hg (C). Solid lines represent the mean difference between the 2 methods, and the dashed lines denote the limits of agreement (±1.96 SD).
We performed an analysis of variance (Fig. 4) to analyze the effect of the different levels of PaCO2 on the bias from the blood samples and the corresponding PtcCO2. The difference between both variables increases in line with PaCO2 severity: level 1, 1.77 ± 3.29 mm Hg; level 2, 3.69 ± 2.84; level 3, 6.77 ± 4.75 (analysis of variance, P < .001). During this study, we did not observe any adverse effects from or subject discomfort with the heating electrode, and no skin lesions were found after probe removal.
Analysis of variance test to analyze the bias related to PaCO2-PtcCO2 couple at different levels of PaCO2.
Discussion
The present study evaluated the accuracy of the SenTec digital monitor for PtcCO2 measurements in subjects with acute respiratory failure according to the severity of hypercapnia. Although we generally found good agreement between transcutaneous carbon dioxide and PaCO2, the measurements of PtcCO2 were less reliable in subjects with severe hypercapnia. In fact, in the group of subjects with higher PaCO2 values, the bias between PtcCO2 and PaCO2 was clinically relevant and could interfere with the interpretation of the results and the management of the respiratory failure.
Although several studies that have investigated the accuracy of PtcCO2 monitoring in subjects with acute respiratory failure have found a good agreement between the 2 methods, most of them did not evaluate patients with high hypercapnia levels. For instance, in subjects admitted to an emergency department, Delerme et al5 found that the mean difference between PaCO2 and PtcCO2 was 1 mm Hg with limits of agreement of −3.4 to 5.6 and that all PtcCO2 values were within 5 mm Hg of PaCO2 values. In subjects with severe asthma or pneumonia, Perrin et al11 obtained a bias of −0.13 mm Hg with limits of agreement of −3.9 and 3.7 mm Hg. McVicar and Eager12 found a mean difference of 0.15 mm Hg, and the limits of agreement were −6 to 6.2 mm Hg. It should be noted that mean PaCO2 in these 3 studies was <50 mm Hg (39, 36, and 41 mm Hg respectively). Conversely, ours was 59 mm Hg, and 48% of the cases were in the group of PaCO2 > 60 mm Hg. This could explain why, in a similar setting of acute respiratory failure, our bias and limits of agreement are worse than those reported previously.
To our knowledge, this is the first study that has purposely assessed the validity of transcutaneous measurements in subjects with different levels of hypercapnia, including a high number of severe hypercapnic patients. According to our results, the difference between the 2 variables increases as the level of PaCO2 rises. The group with PaCO2 > 60 mm Hg showed a bias of 6.7, with limits of agreement of −2.5 and 16. Our findings are consistent with those of other authors,13,14 who have argued that the reliability and accuracy of this method decreases when evaluating severe ventilatory disturbance with high levels of PaCO2. Storre et al,13 who used a SenTec digital monitor, reported a bias of 4.6 mm Hg and limits of agreement of −3.9 and 13.2 mm Hg in 10 subjects with a mean PaCO2 of 67 mm Hg. Kelly and Klim,9 using a Radiometer TCM4 device in subjects undergoing noninvasive ventilation, found a poor agreement between PaCO2 and PtcCO2. In this study, mean PaCO2 was 60 mm Hg, and the bias was 6.1, with limits of agreement of −10 and 22 mm Hg.
In contrast, in a study designed to assess the accuracy of a TOSCA monitor in ICU subjects with varying PaCO2, Bendjelid et al6 concluded that the agreement between the 2 methods was independent of the level of PaCO2. However, the study did not discriminate high PaCO2 values because the hypercapnic group was defined by PaCO2 > 42 mm Hg. In addition, the authors did not mention the average level of PaCO2, but when their graphs are analyzed, it seems that most samples had a value of <60 mm Hg. Cox et al,7 using the same monitor as Bendjelid et al6 in subjects with exacerbation of COPD, also obtained a good correlation between PaCO2 and PtcCO2. However, despite finding a small bias (mean PtcCO2 – PaCO2 difference of −1.1 mm Hg), the limits of agreement were relevant (+4.5 to −6.8 mm Hg).
In accordance with other authors,9,14,15 another important fact that we found was that PtcCO2 values underestimate PaCO2 values. We observed that not only does PtcCO2 underestimate PaCO2, but also, this underestimation becomes more pronounced as hypercapnia increases. These results are very important in clinical practice because they can give a false impression of normality, especially in patients who may require ventilatory support. Our study was carried out in a respiratory intermediate care unit, where patients with severe ventilatory failure/disturbance and severe hypercapnia predominate (COPD exacerbation, neuromuscular diseases, obesity hypoventilation syndrome). In this context, although PtcCO2 is useful to avoid repeated arterial blood samplings, PtcCO2 values need to be interpreted with caution, especially in cases with high levels of hypercapnia, and arterial blood gas analysis therefore remains the accepted standard in the management of these patients. In critically ill subjects as Rodriguez et al16 suggest, transcutaneous arterial carbon dioxide is probably more useful as a trend-monitoring tool to predict changes in PaCO2 than as a single measurement. In the same way, van Oppen et al17 in a pilot study demonstrate that PtcCO2 provides a continuous and reliable tool and also allows pH prediction.
Some monitor and patient factors have been described that may affect transcutaneous carbon dioxide measurements and therefore could alter the results of our study. In order to avoid technical problems like trapped air bubbles, improper placement, or inappropriate calibration, the monitor was only used by trained personnel. Body mass index, tissue hypoperfusion, and the use of vasoconstricting drugs may affect accuracy.18,19,20 In line with the results of Maniscalco et al,21 which found a good agreement between PaCO2 and PtcCO2 (bias 1.3 mm Hg and limits of agreement −4 to 1.1) in a study of severe obese subjects (body mass index 43.7 kg/m2), we considered that the body mass index of our subjects (mean 34 kg/m2) did not affect the accuracy of PtcCO2. On the other hand, only 2 hypotensive subjects were included.
This study has some limitations that should be considered. PtcCO2 measurements were recorded in real time, and the values were not retrospectively analyzed with the software provided by the SenTec digital monitor to correct hypothetical drift. In any case, the drift of the transcutaneous electrode would be relevant if monitoring is performed over a period of several hours22,23 and not during short-term studies like ours, so we considered that this would not alter our results. The classification of PaCO2 values in 3 different groups was done arbitrarily. Therefore, we are unable to establish a cut-off point corresponding to a PaCO2 level at which the difference is clinically unacceptable. Our study was not designed to assess the accuracy of the monitor when used to follow trends in PaCO2 over time because only one comparison was made. However, other authors16,17 consider PtcCO2 monitoring a useful tool in the treatment of acutely ill subjects. In these cases, measurements of PtcCO2 would complement the arterial samples for following and monitoring trends.
Although we have evaluated a single-monitor device and consider that the level of agreement is related to the severity of hypercapnia and not to the accuracy of the SenTec digital monitor itself, further studies with different monitors and different levels of hypercapnia are probably needed to confirm our results.
Conclusions
Our study suggests that in subjects with acute respiratory failure, a portable PtcCO2 device provides an acceptable assessment of PaCO2 when compared with the accepted standard measurement of PaCO2. However, these findings show that we have to take into account 2 clinically important aspects: PtcCO2 undervalues PaCO2 levels, and PtcCO2 is less reliable in patients with severe hypercapnia. Bearing in mind these limitations, we cannot recommend the use of PtcCO2 monitoring as a substitute for arterial blood gas analysis, especially in severely hypercapnic patients who could potentially require ventilatory support.
Acknowledgments
We acknowledge the contribution of Dr Planas, Elena Garcia, and the rest of the nursing staff of the Respiratory Department (Hospital Universitario de Bellvitge) to data collection for this project.
Footnotes
- Correspondence: Yolanda Ruiz MD, Feixa Llarga s/n, 08907 Hospitalet de Llobregat, Spain. E-mail: yolanda.ruiz{at}bellvitgehospital.cat.
Dr Prats presented a version of this paper at the European Respiratory Society 2014 annual meting, held September 6–10, 2014, in Munich, Germany.
The authors have disclosed no conflicts of interest.
- Copyright © 2016 by Daedalus Enterprises