Abstract
Intermittent mandatory ventilation (IMV) was introduced nearly 50 years ago. Despite the initial fanfare and early adoption by many, the role of IMV continues to be questioned. The use of small tidal volumes complicates the application of IMV, and issues with work of breathing, weaning and lack of clear advantages have many calling for a moratorium on its use. Spontaneous breathing, however, has a number of salutatory effects on gas exchange, the distribution of ventilation, and hemodynamics. These issues will be explored in light of a growing body of evidence.
- Mechanical ventilation
- intermittent mandatory ventilation
- synchronized intermittent mandatory ventilation
- asynchrony and work of breathing
Introduction
Intermittent mandatory ventilation (IMV) was first described in 1971 for use in pediatric patients by Kirby et al1,2 and subsequently in 1973 by Downs et al3,4 for use in adults. IMV is a mode of ventilation where intermittent mandatory breaths are delivered at clinician-defined intervals, and between these mandatory breaths, the patient can breathe spontaneously or with pressure-supported breaths.5 The initial pediatric application was associated with the development of a ventilator using various Bird Corporation parts, which was eventually referred to as the Baby Bird (Fig. 1).1,2 For adults, IMV did not initially result in a commercial product but an approach to modify existing ventilator circuits to allow spontaneous breathing between mandatory breaths (Fig. 2).3,4 The question being addressed in this pro/con discussion is: Has the time come for IMV to be retired from the list of available modes of ventilation, since other modes perform at least as well as IMV, there are no documented advantages to the use of IMV, and there are potential disadvantages?
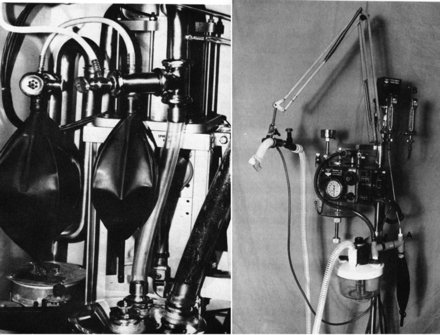
The first pediatric intermittent mandatory ventilation ventilator, the Baby Bird.
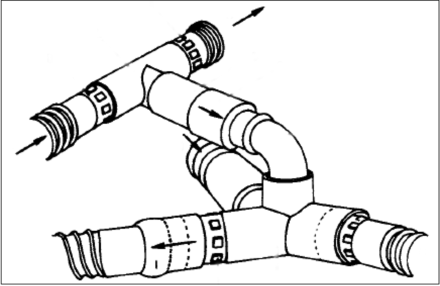
Assembly used to convert a standard ventilator to an intermittent mandatory ventilation circuit. A high flow of gas moves across the Y-piece of the ventilator circuit. The 2 circuits are separated by a one-way valve only allowing flow into the ventilator circuit by active patient inspiration. From Reference 3, with permission.
The Beginning
At the time that IMV was introduced into adult mechanical ventilation, the only ventilators available for use in the ICU were intermittent positive-pressure breathing type pressure targeted ventilators and ventilators with volume control designed to only provide controlled mechanical ventilation.6 Controlled mechanical ventilation on these units did not function as today's ICU ventilators. There was no possibility of the patient triggering a breath.6,7 Although many used intermittent positive-pressure breathing devices to provide continuous ventilatory support, the majority of these applications failed. Thus, the primary ventilators only provided controlled ventilation. As a result, patients either had to conform to the ventilator output as set by the clinician or be sedated and frequently paralyzed in order to tolerate ventilatory support. There was a need to provide some method of allowing the patient to interact with the ventilator in a more synchronous manner, and IMV filled that need. As a result, almost every ventilator on the market was refitted to allow the application of IMV (Fig. 3). Over time, manufacturers incorporated IMV into adult ventilators with volume control mode in the form of synchronized IMV (SIMV).8 With this approach, mandatory breaths were synchronized to the patient effort.5 The first ventilator that allowed assist-control ventilation (continuous mandatory ventilation [CMV]) was the Puritan-Bennett MA-1, introduced in the late 1960s.6,7 The final step in the developmental process of IMV occurred with the introduction of the Servo 900C, which included pressure support ventilation (PSV).9,10 With PSV, the spontaneous breaths of the patient could be supported, and eventually the mandatory breath could be set as either a pressure-controlled or volume-controlled breath. Over the years, there have been numerous case series11–15 supporting the use of SIMV but, as with many modes of ventilation, little evidence supporting an advantage of IMV over currently available modes of ventilation.
Emerson post-operative ventilator (left) and Bird ventilator (right), each with an intermittent mandatory ventilation circuit added. From Reference 6.
Today there are numerous ventilators that allow intimate patient ventilator interaction using a variety of ventilator modes, all of which have at least the potential of providing as much or more synchrony at a much lower patient work of breathing. In the following sections, it will be demonstrated that IMV lengthens the time of mechanical ventilation, provides no advantage over continuous mandatory ventilation in maintaining patient's acid/base balance, does not provide ventilatory support at a lower work of breathing, and does not result in a greater level of synchrony than other modes of ventilatory support.
Pro: Yes, IMV Should Be Abolished
Liberation From Ventilatory Support
Esteban et al16 and Brochard et al17 published landmark studies during the 1990s demonstrating that IMV was the poorest approach to determining whether patients are ready for ventilator discontinuation. In both of these studies, subjects who initially failed a spontaneous breathing trial were randomized to IMV, PSV, or a spontaneous breathing trial as an approach to determine readiness for ventilator discontinuation. Specific protocols were defined for each approach. With the spontaneous breathing trial approach, subjects had to successfully complete 2 h of spontaneous breathing. With PSV, they had to sustain spontaneous breathing at either 8 cm H2O for 24 h17 or 5 cm H2O for 2 h,16 and with IMV, they had to sustain ventilation with a rate of 4 breaths/min for 24 h17 or 5 breaths/min for 2 h.16 In both studies, the number of days to successful liberation was significantly greater with IMV than with PSV or the spontaneous breathing trial. Subsequent studies of weaning and the use of therapist-driven protocols excluded the use of IMV as a possible approach.18–24 Ventilator discontinuance guidelines do not recommend the use of IMV as an approach to assessing patient readiness for ventilator liberation.25 The most probable reason for these outcomes was that low-level IMV requires higher patient effort during both the mandatory and spontaneous breaths than other approaches to ventilatory support.
IMV and Acid-Base Balance
One of the issues that had been argued for the superiority of IMV over CMV is the ability of IMV to better maintain a patient's acid/base balance. This was reported not to be so by Hudson et al.26 In 36 subjects, they compared acid/base balance during IMV and CMV and found no clinically important differences in pH or PaCO2 regardless of the presence or absence of brain injury. They suggested that although minute ventilation was lower during IMV, carbon dioxide production was increased. Therefore, the pH and PaCO2 did not change.
Work of Breathing
Patients receive mechanical ventilation to reduce their work of breathing. However, it has been shown that as the IMV rate decreases, not only does respiratory drive increase, but the amount of work performed by the patient during both spontaneous and mandatory breaths increases.27–32 Weiss et al27 compared respiratory drive and timing in 7 subjects requiring ventilatory support but capable of breathing spontaneously for sufficient time to complete their study. They found that as the IMV rate was decreased, respiratory timing was not altered, but ventilatory drive increased as measured by peak inspiratory flow and P0.1.
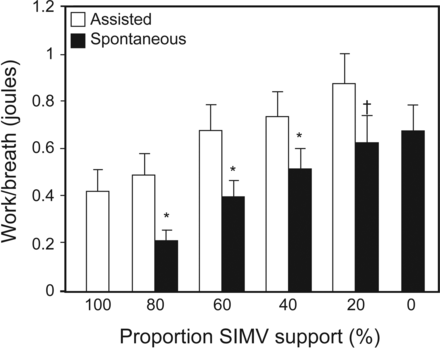
Marini et al28 showed in subjects with COPD receiving ventilatory support that, as the support provided by mandatory breaths decreased, the work performed by the subject increased not only in the unsupported spontaneous breaths but also during the mandatory breaths. Figure 4 illustrates these results. Note that all subjects began the trial on volume control, but after a period of stabilization and measurement, the IMV rate was decreased from 100% (CMV) to 80, 60, 40, and 20% IMV mandatory rate and then to CPAP. As the mandatory rate decreased, patient work during the spontaneous breaths increased, but unexpectedly, the work doing the mandatory breaths also increased to a similar extent.
Mean inspiratory work of breathing during assisted breaths and spontaneous breaths across the spectrum of ventilatory support continuous mandatory ventilation (100%) to CPAP (0). At each level of synchronized intermittent mandatory ventilation (SIMV), the work of breathing increased; however, the work during the assisted breaths was higher than during the spontaneous breaths. Bars indicate SE. * P < .01; † P < .05. Adapted from Reference 28.
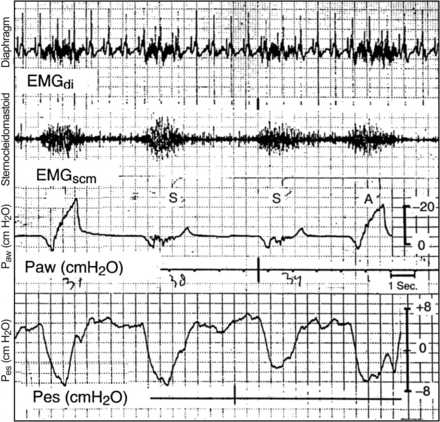
Imsand et al29 studied the neuromuscular output of subjects weaning from ventilatory support who were being managed in volume-targeted IMV. Only when the mandatory rate represented >60% of the total support did the neuromuscular output decrease. At lower mandatory rates, there was no difference in electromyographic activity of the respiratory muscles and the P0.1 between mandatory and unsupported spontaneous breaths. Figure 5 shows the electromyographic signal from the diaphragm (EMGdi), the electromyographic signal from the sternocleidomastoid muscle (EMGscm), and airway pressure and esophageal pressure change reflective of pleural pressure change during both mandatory and spontaneous breaths. Note that this subject is making the same effort during the mandatory breaths (esophageal pressure change and EMG activity) as during the spontaneous breaths. Why would this be the case? It appears that the respiratory center is not able to rapidly differentiate load on the respiratory system breath to breath if the load is changing rapidly and if a low level of mandatory breaths is applied compared with spontaneous breaths. As a result, the respiratory center assumes that the load it should respond to every breath is the greater load. Thus, as the ventilatory load increases during the spontaneous breath, it assumes that the increased load is present in all breaths. This becomes particularly troublesome when the spontaneous unsupported rate is ∼50% or greater than the mandatory rate. Down-regulation of the neuromuscular output during IMV only appears to be large when the mandatory assistance is ≥75–80% of the total assistance.29,31
Electromyograms of the diaphragm and of the sternocleidomastoid muscles, showing similar intensity and duration of electrical activity in successive assisted (A) and spontaneous (S) breaths. Paw = airway pressure; Pes = esophageal pressure; EMGdi = electromyographic signal from the diaphragm; EMGscm = electromyographic signal from the sternocleidomastoid muscle. From Reference 29, with permission.
This increased effort is a concern because most apply IMV at the lowest mandatory rate that sustains the patient's gas exchange. However, this rate may be right at the border of fatigue for the patient, preventing the patient from resting and recovering from ventilatory failure. This is probably the explanation for why IMV is the approach to ventilator liberation most likely to result in the longest period of ventilatory support. It is not uncommon to see patients managed with IMV having a set mandatory rate of 6–12 breaths/min with an overall breathing frequency at least twice this level.
PSV applied to the spontaneous breaths decreases the work of both the spontaneous breaths and the mandatory breaths.33 It appears that unloading the spontaneous breath alters the memory phenomenon observed with unsupported spontaneous breaths.34 However, adding pressure support changes the concept of IMV. Does the combination of the 2 different gas delivery approaches make it more difficult to liberate patients from ventilatory support? To compensate for the increased work of breathing during the mandatory and spontaneous breaths, SIMV has been used as pressure control of 15 cm H2O for the mandatory breaths and pressure support of 15 cm H2O for the spontaneous breaths. What is the patient receiving: SIMV, pressure control, or PSV? There is no benefit to managing a patient in this manner compared with using PSV or CMV.
Similar data exist in the pediatric literature. Kapasi et al30 compared work of breathing during pressure-controlled IMV, pressure-controlled SIMV, PSV, and pressure control in 7 newborns. These children were 31.4 ± 2 weeks gestation and 1.49 ± 0.38 kg. The ventilator settings determined by the managing team were maintained unchanged; all that was changed was the mode in a randomized fashion, each applied for 20 min. They found that the work of breathing was markedly greater during IMV and SIMV than during PSV and pressure control. Beck et al32 measured EMG activity and neural timing of mandatory breaths and unassisted spontaneous breaths in preterm infants who were not sedated. They found that the EMG activity was similar regardless of breath type.
Asynchrony
Recently, increasing emphasis has been placed on asynchrony and its effects on patient outcome.35–40 The presence of an asynchrony index (all asynchronous breaths divided by total breaths, triggered, and missed triggered breaths times 100) >10% has been shown to be associated with an increased length of mechanical ventilation,35,36 ICU stay,35,36 and mortality.37 Mode of ventilation impacts asynchrony, with greater levels of mechanical assistance resulting in greater levels of asynchrony.38 However, until recently, little data on the presence of asynchrony during IMV has been available. Robinson et al41 demonstrated that subjects receiving IMV compared with other modes of conventional ventilation had a higher asynchrony index. Of the 35 subjects studied, 9 (25.7%) had an asynchrony index >10%. Subjects were a median age of 47 y, 77.1% were male, 16% had COPD, and their median injury severity score was 22. As a result of the small sample size, there were no differences in ventilator days, ICU days, hospital days, discharge home, and mortality. However, all of these values trended higher in the group with an asynchrony index >10%. Thus, the lower the IMV rate, the greater the patients' work of breathing,29,31 and the higher the IMV rate, the greater the asynchrony index.41 Regardless of how one attempts to modify the application of IMV, there are problems associated with its application.
Use of IMV
Esteban et al42,43 published epidemiologic studies on the worldwide prevalence of modes of mechanical ventilation. In 2000,42 the worldwide use of IMV as a support mode was 7.9% (United States, 13.9%), and its use as an approach to weaning was 6.8% (United States, 5.1%). In 2008, Esteban et al43 indicated that the worldwide use of IMV had decreased to 1.6%. Criticisms of IMV44,45 appear to be on point in abandoning the use of IMV.
Pro Summary: Abolish IMV
IMV does not have any clear advantages over any of the other modes of ventilation. Its reason for existence, allowing spontaneous breathing between mandatory breaths, does not exist today. IMV does not improve gas exchange; it does not decrease the work of breathing and asynchrony; and, most importantly, it does not decrease the length of mechanical ventilation when used as an approach to weaning from ventilator support. IMV has helped clinicians to understand mechanical ventilation and patient-ventilator interaction, perhaps more than any other mode of support. IMV has been instructive, but the lessons are learned.
The Con Position
A Question of Intent
IMV was introduced in the early 1970s1,2 as an alternative to CMV. But beyond discussions of work of breathing and reduced weaning times, the emphasis and advantages of IMV can be traced directly to the maintenance of spontaneous breathing. In fact, the majority of the proposed advantages of IMV arise from the positive attributes of spontaneous breathing. These include improved synchrony, a reduction in the need for sedation, prevention of respiratory alkalosis, reduced air-trapping, improvement in ventilation perfusion matching (V̇/Q̇), a reduction in barotrauma, reduced respiratory muscle atrophy, and improved venous return augmenting cardiac output and improving renal perfusion.46
It would also be remiss not to judge the introduction of IMV in light of the standard of care at the time. Tidal volumes of 12–15 mL/kg were the norm, and even patient-triggered volume control breaths required a significant effort by the patient. The idea to interpose spontaneous breaths from a continuous flow source with mandatory breaths 6–10 times/min, acting as true sigh breaths, seemed a plausible solution to constant flow volume-control ventilation and heavy sedation or paralysis. The interest was sufficient that an entire issue of International Anesthesiology Clinics was devoted to the topic of IMV in 1980.47
One suspects that Kirby and Downs,3,11 faced with the current paradigm of patients ventilated at >20 breaths/min, would lament the complete misunderstanding of the intent of IMV (ie, the lowest possible mandatory rate to support a patient with hypoxemic respiratory failure with PEEP maximized to restore lung volume and place the lung on a portion of the pressure volume curve that reduced the work of breathing).3,11 Weaning was rarely needed in this paradigm because the patient was on the lowest mandatory rate, as early as possible. It should also be noted that the supporters of IMV, very early on, used a room air CPAP trial to determine the suitability for extubation.48
The allure of IMV lives on in 2016 under other names: adaptive support ventilation, airway pressure release ventilation, and biphasic ventilation, modes that my colleague has described as potentially advantageous.6,49–51 Each of these is just another method to exploit the advantages of spontaneous breathing outside the IMV brand.
The Importance of Spontaneous Breathing
Distribution of Ventilation and Gas Exchange.
Froese and Bryan52 published the seminal work on the importance of spontaneous breathing on the distribution of ventilation in 1974. In a remarkable trial of volunteers (“..three volunteer anesthetists of widely differing configuration, who were fully informed of the nature of the proposed study”), they studied the position and movement of the diaphragm during spontaneous breathing and mechanical ventilation during muscle paralysis. The widely differing configurations included a range of body mass index from 23 to 29 kg/m2.
This work demonstrated that spontaneous breathing in an awake or anesthetized state resulted in the greatest displacement of diaphragmatic movement in the dependent position. This occurred despite the fact that intra-abdominal pressure was highest in the dependent lung. Following paralysis and positive-pressure ventilation, the diaphragmatic displacement was reversed. Movement of the diaphragm was cephalad with the largest change in the non-dependent lung regions where intra-abdominal pressure was the least. Importantly, neither an increase in tidal volume nor the application of PEEP was able to correct the maldistribution of lung volume. This is often explained as the distribution of ventilation, following the path of least resistance. The phenomenon is more complicated than that, since gravity, regional lung compliance, pleural pressure gradients, and variations in expiratory lung volumes all contribute to the resulting distribution of ventilation.53,54
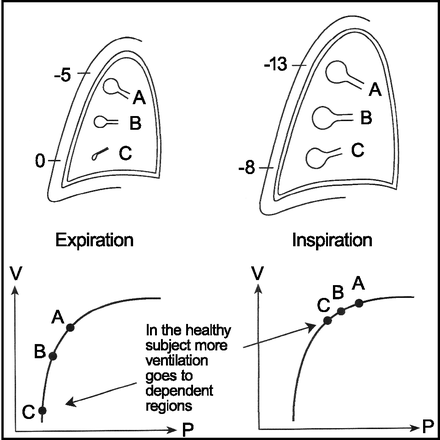
Regional differences in gas distribution are demonstrated in Figure 6. Under normal conditions, a greater volume of inspired gas is distributed to the dependent lung regions compared with non-dependent lung units. Uneven distribution is the result of the normal curve of the lung pressure-volume relationship. Transpulmonary pressure decreases from the apex to the base of the lung. Non-dependent lung regions have higher transpulmonary pressure than dependent lung regions, such that during inspiration, pleural pressure is reduced and dependent lung regions have a larger volume change for a similar transpulmonary pressure. In the mechanically ventilated patient, maldistribution of volume is exacerbated by heterogeneous lung disease (eg, ARDS), supine position, age, sex, body mass index, and paralysis.46
Schematic drawing of alveolar sizes at upper (A), middle (B), and lower dependent (C) lung regions at end expiration and end inspiration. Note the pleural pressure that approaches 0 in the lower part of the pleural space and the more negative pressure higher up. Lower panels show corresponding pressure (P)-volume (V) curves. From Reference 46, with permission.
Following their pioneering work in volunteers, the Canadian group55 studied children undergoing anesthesia for a surgical procedure. They compared the dead space/tidal volume ratio (VD/VT) during 3 methods of airway control and ventilation. Subjects were intubated and received controlled ventilation, were intubated with spontaneous breathing, or received mask anesthesia with spontaneous breathing. The study demonstrated that increases in tidal volume with positive-pressure ventilation failed to alter VD/VT, suggesting greater dead space from overdistention of already ventilated lung regions. Only during spontaneous ventilation did VD/VT fall as breathing frequency slowed and tidal volume increased, suggesting improved matching of ventilation to areas of perfused lung.
Experiments in a porcine model have further defined the advantages of spontaneous breathing in enhancing ventilation perfusion relationships and oxygenation.56,57 Using both computed tomography and a γ camera technique, one group demonstrated that lung injury with oleic acid resulted in a predictable fall in end-expiratory lung volume and oxygenation. Animals were either maintained on controlled ventilation or allowed to breathe up to 30% of the total minute volume spontaneously. The spontaneously breathing group had a marked increase in end-expiratory lung volume, a reduction in the amount of unaerated lung tissue, and a significant improvement in oxygenation. Measurements of V̇/Q̇ also improved as a consequence of greater dependent lung aeration, where gravity-dependent blood flow is greatest.
A schematic representation of the improved distribution of ventilation in the supine mechanically ventilated subject is shown in Figure 7. This is based on the work of Froese and Bryan52 and that of Roussos et al.58 Roussos et al58 demonstrated that with the active diaphragm (his term was “tensed”), the differences in pleural pressures between non-dependent and dependent lung regions was smaller. As a result, the positive pressure seen with a relaxed diaphragm promotes alveolar collapse, whereas maintenance of diaphragmatic activity promotes lung aeration.
Schematic of mechanisms behind the better recruitment of alveoli with spontaneous breathing. The left panels show the excursion of the diaphragm during mechanical ventilation and spontaneous breathing. Note the greater excursion in dorsal parts with spontaneous breathing. The right panels show the difference in the vertical pressure gradient during mechanical ventilation and spontaneous breathing, with a larger variation with mechanical ventilation and the potential of developing positive pleural pressure in the lower parts. This will promote collapse of alveoli. From Reference 46, with permission. Exp = expiratory; insp = inspiratory.
A number of animal trials have all demonstrated the positive impact of spontaneous breathing on V̇/Q̇ and gas exchange.59–62 These investigations were followed by clinical studies in subjects, demonstrating similar results.63–65 In each instance, the maintenance of spontaneous breathing resulted in improved gas exchange resulting from better V̇/Q̇ matching.
With respect to IMV or SIMV and the ability to improve gas exchange and promote recruitment of dependent lung regions, there can be no argument. Compared with CMV, maintenance of spontaneous breathing during IMV provides an anatomic and physiologic advantage.
Cardiovascular Advantages.
Certain physiologic principles are unchanged by opinion or bias with respect to positive-pressure ventilation. Preeminent among these is that positive pressure increases intrathoracic pressure and reduces venous return, resulting in a decrease in preload and a fall in cardiac output.66 Downs and others11,47 surmised that replacing mandatory breaths with spontaneous breaths would counteract this complication and, simply by reducing intrathoracic pressure, improve circulatory performance.
In a number of experimental61,62 and clinical studies,63,65–69 the reduction in intrathoracic pressure through the addition of spontaneous breathing has demonstrated improved venous return, enhanced cardiac output, and increased oxygen delivery. With IMV, this improvement results not only from simply reducing the rate of positive pressure breaths but from maintaining the intrathoracic pump mechanism whereby negative pressure generated during inspiration enhances venous return regardless of the level of PEEP.70,71 There are no comparisons with CMV in the literature that fail to demonstrate the superiority of IMV in improving cardiac performance. By maintaining improved cardiac output, IMV may reduce the need for fluid resuscitation and the use of vasopressors and decrease fluid accumulation in the lung seen with aggressive resuscitation.
Liberation From Mechanical Ventilation
Although IMV was in fact introduced as a method to facilitate weaning,3,72,73 the intended application of IMV in patients with hypoxemic respiratory failure was that weaning would begin on day 1 (ie, the lowest IMV rate that kept the spontaneous breathing frequency <30 breaths/min was tolerated).
Studies evaluating IMV as a weaning technique have been inherently flawed by the arbitrary slow reduction in breathing frequency in 1–2-breath increments in a predetermined time frame.16,17,74 Patient selection is also important. Whereas Downs et al4 and others described the use of IMV for weaning in patients with chronic lung disease, our current understanding of respiratory muscle physiology, muscle fatigue, and air trapping suggests that IMV should be avoided in this population, although by replacing mandatory breaths with spontaneous breaths, air trapping may be diminished, and the patient can become more comfortable and synchronous. The ideal patient for initial ventilatory support and then termination of ventilatory support through a spontaneous breathing trial is the patient with hypoxemic respiratory failure, low lung volume, and an absence of preexisting chronic respiratory dysfunction.
Acid-Base Balance
One early proposed advantage of IMV was the avoidance of respiratory alkalosis.3,4 Respiratory alkalosis can occur with CMV and pressure support, resulting in periodic breathing and disturbed sleep cycles.75–77 The paper by Hudson et al26 evaluated subjects with respiratory alkalosis on CMV and transitioned subjects to IMV at half the assist control rate. Minute ventilation was lower with IMV, but CO2 production was increased, resulting in equivalent values for PaCO2. This is another study accomplished when tidal volumes of 10–15 mL/kg were common. There was a modest but statistically insignificant fall in pH (from 7.51 to 7.48). The maintenance of the respiratory alkalosis following transition to IMV is troubling and perhaps was due to insufficient PEEP in the study by a group of investigators known to be cautious with the use of PEEP at that time. Failure to use PEEP in appropriate amounts to help reduce the work of breathing could have led to continued tachypnea. Although ignored in the early pages of this paper, Gallagher78 did demonstrate frequent respiratory alkalosis, complicating weaning from mechanical ventilation with CMV.
The Work of Breathing
Whereas the points earlier in this paper focus on the work of breathing during a single breath or from breath to breath, research in this area fails to acknowledge that simply increasing the mandatory IMV rate, thereby reducing the percentage of the minute ventilation required via spontaneous breathing, in fact reduces the work of breathing. Work of breathing during IMV is also impacted by choice of the patient population, appropriate application of PEEP, system for IMV delivery, and appropriate use of mandatory breath rate and size to assist in lung inflation and carbon dioxide removal.
IMV augments spontaneous breathing with mandatory breaths, which act as a sigh to remove carbon dioxide and prevent alveolar collapse. Paramount to the success of IMV is patient selection (ie, a patient with low lung volumes, tachypnea, hypoxemia, and normo- to hypocapnia). Under these conditions, the judicious use of PEEP to restore end-expiratory lung volume and position the patient on the steep portion of the pressure-volume curve allows work of breathing to be managed.79 Application of IMV in patients with respiratory muscle fatigue, chronic lung disease, or neuromuscular disease is a misapplication of the technique. In these cases, a mode of support that rests fatigued muscles is called for. The failure of IMV in this instance is a failure of application.
Perhaps the worst problem to befall IMV was the introduction of intermittent demand ventilation (IDV) or demand valves for spontaneous breathing.8 This initial investigation consisted of several physicians breathing on a modified MA-1 with the ability to provide IDV (SIMV) versus IMV. All of the physicians in the study agreed that the IDV (SIMV) was more desirable, not exactly translational science. In the ensuing years, evaluations of the work of breathing of SIMV systems demonstrated significant advantages of continuous flow systems.80–84 In fact, the 2 authors of this paper have spent good parts of our academic careers comparing the work of breathing imposed by ventilators. And although modern day ventilators have sophisticated systems for triggering and cycling the spontaneous breath, asynchrony in timing remains an issue. Continuous flow systems could complicate monitoring, obfuscate humidifiers of the day, augment tidal volume, and complicate setup. However, the early switch to demand valve systems was not an advantage for the patient, just for the caregiver. Of note, the only investigation to evaluate the role of synchronizing mandatory breaths during IMV failed to show any advantage of SIMV over IMV.85
Asynchrony
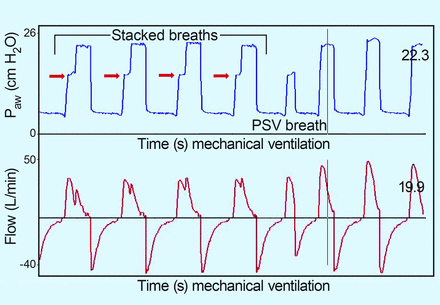
Asynchrony is a common problem during CMV, PSV, and IMV.35,36,41 However, the incidence of asynchrony during assist control demonstrated by Thille et al35 and SIMV as reported by de Wit et al36 is essentially the same. In both cases, missed triggers predominate, a function of patient population (COPD) and excessive ventilatory support. The paper by Robinson et al41 evaluating asynchrony in trauma subjects, many receiving SIMV, finds a similar incidence of asynchrony with none of the negative consequences (prolonged ICU stay, mortality, etc). More than half of our asynchrony events were stacking of adaptive pressure breaths during SIMV + PSV. Figure 8 demonstrates this phenomenon. The opinion of the authors was that this is unique to this ventilator and this mode and that in the volume control mode, this would not happen. The only factor to correlate with this type of asynchrony was the set mandatory rate. The higher the mandatory rate, the greater the risk of asynchrony. Downs and Kirby would probably admonish us to be true to the original principles.
Screenshot of breath-stacking of mandatory breaths on top of spontaneous breaths during adaptive pressure ventilation in the synchronized intermittent mandatory ventilation mode. Note that since breaths are pressure-limited, the absolute pressure is limited but inspiratory time is prolonged; hence, volume is increased. PSV = pressure support ventilation.
Use of IMV
Although the use of IMV is declining42,43 precipitously, it is interesting to note that at one time, IMV was the most frequently applied mode for weaning in the United States.86 Interestingly, the likelihood of being ventilated with IMV correlates with geography. If you live in the United States, you are more likely to be placed on IMV. IMV lives on in other modes (adaptive support ventilation, airway pressure release ventilation, and biphasic ventilation), each maintaining spontaneous breathing as an important feature. The calls for the removal of IMV are premature. Routine use of small tidal volumes, which represent the standard of care, however, render the concept of IMV less attractive, since the use of mandatory breaths as “sighs” no longer fulfills that role at 6 mL/kg.
Outcomes
Every head-to-head comparison of IMV and CMV has concluded that there are no differences in outcomes.13,15,87–89 The truth of the matter is that in large trials, IMV is as useful as CMV, hardly a mandate to eliminate the use of IMV. Perhaps a re-evaluation and re-education of the appropriate application of IMV is the real mandate.
Conclusions
In a paper, such as this, where the pro and con must be defended with vigor, regardless of the viewpoints of the authors, the evidence for the continued use of IMV or SIMV is on life support. Spontaneous breathing has many advantages. In 2016, however, these advantages can be obtained through other techniques, such as proportional assist ventilation (PAV) and neurally adjusted ventilatory assist (NAVA), while better supporting the patient's work of breathing. IMV clearly demonstrates no evidence to speed ventilator discontinuation. In patients with respiratory muscle fatigue or respiratory muscle dysfunction, IMV may, in fact, prolong weaning, bringing back the days when IMV was referred to as “intermittent respiratory failure.” Our practice must be informed by evidence, and at present the evidence for continued use of IMV outside of very special circumstances cannot be supported.
Discussion
Hess:
The one place where IMV might have a role, as you pointed out in your last slide, is to give a periodic sigh. There are now a number of papers, one is a paper in Critical Care Medicine by Mauri et al,1 who use biphasic positive airway pressure every 0.5–2 min to raise the pressure up to 35 cm H2O for 3–4 s. It's a variation on IMV or airway pressure release ventilation or both of those things. They report that it improves alveolar recruitment, shunt, oxygenation, and so forth. I can say anecdotally that I've tried it in a few patients and was underwhelmed, but it might be something we should keep in mind.
MacIntyre:
I wrote the editorial for that paper1 and they gave an awful lot of these sigh breaths. When they were doing 2 sigh breaths/min, they were providing 34 or 35% of the minute ventilation with VT levels and pressures that most of us would consider excessive. That worried me a lot. They had very low PEEP levels in that trial. I think if you have a low PEEP situation, recruitment maneuvers and sigh breaths are going to look a lot better than if you had a more aggressive PEEP strategy.
Hess:
Which might explain why I have been underwhelmed the few times I've tried this.
Branson:
The guys who invented IMV considered those 2 or 3 breaths/min as a sigh to recruit the lung. They weren't really looking at it as a method to maintain minute ventilation.
MacIntyre:
When the number of sighs exceed 50% of the minute ventilation is it still a sigh?
Branson:
I think there's a lot to that issue. The use of these large volume breaths and I've heard the IMV guys say the average frequency in the ARDSNet trial2 was 35 breaths/min. That's 50,000 breaths/d. If you're on an IMV rate of 6, you have fewer than 10,000 breaths in a day. Somebody showed yesterday that it's not just the stretch but how many times you stretch the lung that matters. What if you had an approach that did a sigh twice a minute but the rest of the time you had low VT and aggressive PEEP? Clearly, we've already seen the history of this mechanical ventilation idea if you're in the OR. If and they notice during chest surgery that the FRC (functional residual capacity) falls and say let's give a sigh breath to reverse this problem. The sigh breath described was the anesthesiologist grabbing the bag and squeezing it with both hands up to a pressure of 40 (cm H2O) and holding it there for a minute. That translated into low VT levels, consistent monotonous VT levels are bad. In the OR, you get 10–15 mL/kg, and that migrated over into the ICU. Well, when you're in the ICU at 10–15 mL/kg, giving a sigh breath was a waste of time because virtually every breath was a sigh breath. Now that we've gone down to 4–6 mL/kg, the sigh breath may now be useful again. It's all about how we manage the airway pressures.
MacIntyre:
Depending on—
Branson:
Yes. PEEP, breath size, it all matters in whether a technique does or doesn't work.
Marini:
It is true that when comparing the triggered breath with the surrounding spontaneous breaths at any given level of SIMV, the efforts are very similar. And that probably gives rise to asynchrony; one time you get a big breath of fixed length, and the next time you're pretty much on your own or with some lower level of pressure support. But as you give more and more machine-set breaths/min, the vigor of breathing diminishes. It can be an effective weaning mode if used carefully in an automated way. I'm not advocating this, I'm just saying that it's been suboptimally used in some of the published work. And I certainly agree with the sighing and disruption of monotony that it provides. SIMV is occasionally helpful for patients who cannot be ventilated easily because they have unstable drive to breathe—for example, awakening from sedation, when some get chaotic and then they fall asleep. You then have a very wide variation in ventilatory drive. Periodically giving 4 SIMV backup breaths tends to make the patient a little safer to manage—at least they don't go from all to nothing at all. The last thing I'd say is that variation in breath size might be helpful, at least theoretically. You alluded to this, Rich. The monotonous, consistent breath support that we give may not be natural. We may periodically need variation to keep our lungs healthy and uniformly recruited. You can call it biologically variable ventilation, or whatever, but SIMV kind of mimics that if you think about it, providing some bigger breaths and some smaller breaths. It therefore could have a positive physiologic rationale. Again, I don't advocate SIMV for weaning—we have better options. But it can be used effectively in that way, and it may have some advantages in highly selected situations. I don't think we ought to throw it out completely.
Kacmarek:
Why not put those patients on PAV or NAVA and let their respiratory center dictate the variability instead of us trying to select for them what VT and what frequency that variability should occur? I agree there are patients, as you've said, but we have modes of ventilation that do a much better job today than SIMV in accommodating that type of breathing pattern.
Marini:
Point well taken. If you have a ventilator that can provide NAVA or PAV+, you may do well with that. I still think you have the question ofhow do you back those people up when they're on PAV or NAVA.
MacIntyre:
You don't have to do NAVA or PAV. Pressure support lets patients pick the VT levels and frequency they want as well, and it's a lot simpler, cheaper, and more readily available.
Kacmarek:
But you're more likely to get the large VT John is referring to with pressure support as compared with PAV or NAVA. Both of them have backup ventilation if the patient goes apneic; you can set it for a short period.
MacIntyre:
You can do that with pressure-continuous mandatory ventilation. Just set the frequency to be 2 or 4 as a backup rate, and the rest are patient-triggered so that they can manipulate the frequency and VT. You're just setting inspiratory time with that mode.
Kacmarek:
What we're both saying is that we have alternate modes that do a better job. And that the problems with SIMV outweigh the potential benefit of utilizing it—for me, in any patient—today.
Marini:
They require less surveillance than SIMV, but there's nothing intrinsically wrong with SIMV other than that fact that you have to be there to manage and monitor it. I think advocates are used to doing that.
Kacmarek:
The reality at the bedside is there's not that moment-to-moment monitoring.
Marini:
We don't use it either, only very rarely in selected patients.
Kacmarek:
They still use it in cardiac surgical patients, although we've gotten them to move to pressure support as soon as the patient starts making aggressive inspirations. But they come out of the OR on SIMV all the time. In our neonatal unit, even though we studied it in that same neonatal unit, they still use SIMV.3
Kallet:
I absolutely agree, I think one of the things we're saying is it's not necessarily the mode. The problem is how the mode is used. I'm not quite as old as you guys, but I came into Respiratory Care during the early years of IMV. If I recall, the year that IMV was invented was also when you had the assist mechanism on IMV—they came up with that in the ‘70s. So at the same time that they allowed for assisted mechanical ventilation was the time when other clinicians were saying we need to do something different than this.
Kacmarek:
When did the MA-1 come out?
* Masferrer:
1967.
Kacmarek:
That was the only ventilator with true assisted ventilation.
Kallet:
I think it took them a few years to adapt the MA-1 to have that mechanism. We use IMV for patients with a low minute ventilation demand but who are very alkalotic. There are very few patients where it works well. We use intermittent mandatory ventilation for patients who have a respiratory drive that is variable or drug overdose, and it prevents the alarms from going off. That's a solution for a small percentage of patients. To reiterate, I think a lot of it is how the mode is used. Two breaths every 4 h it got worse when we added pressure to that, where the pressure support breaths have higher pressure and larger volume than the IMV breaths. In terms of weaning, it was how clinicians were using it more than the mode. One of the greatest quips about IMV I heard from John Murry; he called it intermittent mandatory respiratory distress.
Kacmarek:
I think that's a good name for it.
Branson:
My big concern now is I see people with these low VT levels still wanting to use IMV, so they set the IMV frequency at 20. Try to breathe on your own in between 20 breaths/min; it's not very easy. So then you walk up to the ICU and go through their history, and in 3 d, the patient hasn't taken a spontaneous breath. It's called IMV, but it's acting exactly like CMV. That is a major problem.
Footnotes
- Correspondence: Robert M Kacmarek PhD RRT FAARC, Respiratory Care, Warren 1225, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 01460. E-mail: rkacmarek{at}partners.org. Richard D Branson MSc RRT FAARC, University of Cincinnati, 231 Albert Sabin Way, ML #0558, Cincinnati, OH 45267. E-mail: Richard.branson{at}uc.edu.
Dr Kacmarek and Mr Branson presented a version of this paper at the 54th Respiratory Care Journal Conference, “Respiratory Care Controversies III,” held June 5–6, 2015, in St Petersburg, Florida.
Dr Kacmarek has disclosed relationships with Covidien, Orange Medical, and Venner Medical. Mr Branson has disclosed relationships with Bayer, Meiji Pharmaceuticals, Medtronic, Mallinckrodt, Phillips, and Ventec.
↵* Ray Masferrer RRT FAARC, Managing Editor, Respiratory Care.
- Copyright © 2016 by Daedalus Enterprises
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.
- 13.↵
- 14.
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.
- 61.↵
- 62.↵
- 63.↵
- 64.
- 65.↵
- 66.↵
- 67.
- 68.
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.
- 82.
- 83.
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.
- 89.↵