Abstract
BACKGROUND: Tuberculosis (TB) remains an important public health problem worldwide, as its residual lesions result in functional and quality of life impairments. Few studies have investigated multiple-drug-resistant pulmonary TB (MDR-TB), and the literature regarding the functional parameters of this group of patients is scarce. Functional characterization may point to the need for post-treatment intervention measures that optimize the quality of life in patients with MDR-TB. Thus, this study sought to analyze the respiratory function, functional capacity, and quality of life of patients who were treated for MDR pulmonary TB.
METHODS: This study investigated a cross-sectional cohort of MDR-TB patients who underwent drug treatment for at least 18 months. Patients who had associated diseases (human immunodeficiency virus [HIV], severe heart disease, and hypertension) or disabilities that prevented them from walking were excluded. The subjects underwent the following assessments: forced spirometry, a chest radiograph, the 6-min walk test, a bioelectrical impedance analysis, maximal inspiratory and expiratory pressures, and a health-related quality of life questionnaire.
RESULTS: Eighteen patients who met the eligibility criteria were enrolled. Spirometric evaluation showed that 78% of the subjects had abnormal patterns. The maximal respiratory pressures were significantly decreased in all subjects, despite the fact that their nutritional status was within the normal range. The distance completed in the 6-min walk test was less than expected in 72% of the subjects. All of the subjects who were evaluated had residual lesions, and 78% reported a worsening in their quality of life.
CONCLUSIONS: In conclusion MDR-TB cured subjects exhibit impaired respiratory function and a mildly reduced functional capacity and quality of life, suggesting that a portion of these patients may require a pulmonary rehabilitation approach.
- tuberculosis
- TB
- multiple-drug-resistant
- pulmonary
- quality of life
- respiratory function
- pulmonary
- rehabilitation
Introduction
According to the World Health Organization, multiple-drug-resistant pulmonary tuberculosis (MDR-TB) is defined as TB that exhibits resistance to at least rifampicin and isoniazid.1 Brazil is one of the 22 countries that represent 80% of the TB cases worldwide, and the state of Rio de Janeiro presents the highest rates of MDR-TB incidence and mortality in the country.1,2 One of the biggest problems in TB control is treatment abandonment, which increases the number of patients with bacteria that are resistant to the major available drugs.1,2
TB, whether multiple-drug-resistant or not, may generate residual lesions that impair functionality and quality of life.3 Despite this fact, no monitoring and/or treatment programs for such clinical and functional changes currently exist, because public health prioritizes the search for new cases and treatment adherence. Even though the level of worldwide concern regarding TB research has increased in the last few years, there are few studies about MDR-TB, and the literature lacks data on the functional aspects of this group of patients. The functional characterization of these patients may point to the need for future pulmonary MDR-TB post-treatment intervention measures that optimize patients' quality of life. Thus, this study was aimed at analyzing the respiratory function, functional capacity, and quality of life of patients who were treated for MDR-TB.
QUICK LOOK
Current knowledge
Tuberculosis can generate residual lesions that impair functionality and quality of life. Despite this fact, there are currently no monitoring and/or treatment programs for clinical and functional changes, because public health prioritizes the search for new cases and treatment adherence.
What this paper contributes to our knowledge
Patients successfully treated for multidrug-resistant tuberculosis present with impaired respiratory function and a mildly reduced functional capacity and quality of life. These findings suggest that a portion of these patients may benefit from pulmonary rehabilitation.
Methods
This study was performed at the Laboratory of Respiratory Physiology, University of the State of Rio de Janeiro, Rio de Janeiro, Brazil.
This was a cross-sectional cohort study of patients who completed MDR-TB therapy in the clinic for MDR-TB treatment and underwent monitoring between May 2008 and May 2010. The clinic is located at the Instituto Estadual de Doenças do Tórax Ary Parreiras, and the recruited subjects were referred from this location to the pulmonary function laboratory of the Hospital Universitário Pedro Ernesto, Universidade do Estado do Rio de Janeiro, in Rio de Janeiro, Brazil. We included patients with MDR-TB, all of whom developed MDR-TB after abandoning treatment. Secondary MDR-TB was diagnosed through mycobacterial culture, with species identification and sensitivity testing indicating the presence of strains that are resistant to the anti-tuberculostatic regimen. Following the Brazilian Thoracic Association's guidelines on tuberculosis,4 all subjects were treated with 5 drugs in the first 6 months (ethambutol, ofloxacin, pyrazinamide, streptomycin, and terizidone) and 3 drugs in the following 12 months (ethambutol, ofloxacin and terizidone). The subjects met the bacteriological criteria for cured MDR-TB (3 consecutive months with at least 5 negative cultures), and had no specific symptoms of active disease (evening fever, weight loss, sweating).5 None of the subjects had extensively drug-resistant TB (resistance to any fluoroquinolone or at least one of the 3 injectable anti-TB drugs: capreomycin, kanamycin, and amikacin).1 Subjects with positive serology for human immunodeficiency virus (HIV) or acquired immune deficiency syndrome (AIDS), severe heart disease, and conditions that prevented the 6-min walk test (6MWT) were not included. The project was approved by the institutional ethics committee, and all participants signed an informed consent form.
The pulmonary function tests followed the proper biosafety standards and ATS/ERS recommendations.6 Forced spirometry (Collins/GS, Warren E Collins, Braintree, Massachusetts) and maximal respiratory pressure tests were conducted by the physician in charge of our pulmonary function laboratory, using a bacterial/viral filter (Collins DC2, nSpire Health, Longmont, Colorado). The results are expressed in absolute values and as a percentage of the predicted values.7,8 Body bioimpedance analysis was performed with a bioimpedance analyzer (Biodynamic 450, Biodynamics, Seattle, Washington) by the nutritionist in charge of the nutrition laboratory.
The 6MWT was conducted according to the American Thoracic Society (ATS) protocol (2002),9 always by the same examiner, and performed at the same time of the day (morning), in order to avoid both inter-observer and intra-day variability. The equation used as a reference for the 6MWT was the one proposed by Enright and Sherrill (1998).10 Each subject's systemic blood pressure, heart rate, and SpO2 were recorded before and immediately after the 6MWT for safety purposes. The modified Borg Scale was applied before, every 2 min during, and after the 6MWT. The subjects answered quality of life questions from the Airways Questionnaire 20,11 which was always given by the same assessor. The Airways Questionnaire 20 has 20 items with yes/no responses. A 5% value was given to each “yes” response; therefore, the scores ranged from zero (no disability) to 100%. The radiological evaluation of the MDR-TB sequelae was carried out by the pulmonologist in charge of the state referral center for MDR-TB patient treatment. Chest radiograph grading was performed according to the score proposed by Willcox and Ferguson (1989).12
Results
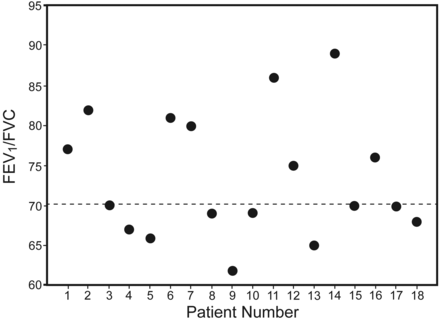
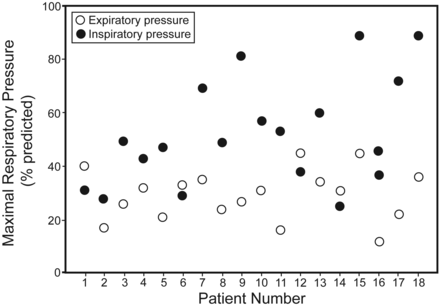
Eighteen consecutive patients (12 male) who met the eligibility criteria were enrolled (Table 1). According to the radiological classification, most of the subjects (n = 11) had grade I disease (minimal involvement of only one zone, without cavitation), 4 subjects had grade II disease (involvement of 2 or 3 zones, or one zone with cavitation), and only 3 of the 18 subjects were classified as grade III (severe involvement in more than 3 zones, with or without cavitation). The forced spirometry results were classified according to ATS/European Respiratory Society (1991).13 The most prevalent pattern was obstructive (39%, Fig. 1), followed by restrictive (22%), normal (22%), and mixed (17%). We did not find an association between the number of pack-years and FEV1 (% predicted) (P = .53) or a statistically significant difference in FEV1 between the smokers and non-smokers (P = .54). A maximal respiratory muscle pressure evaluation revealed results that were low (P < .001), showing values that were, on average, 52.6% and 29.3% of those predicted for maximum inspiratory pressure (PImax) and maximum expiratory pressure (PEmax), respectively (Table 2 and Fig. 2). Most of the subjects (72%) presented with a reduced functional capacity, with, on average, a 6MWT distance that was 15% lower than the expected value (P = .004) (see Table 2). The percentages of the subjects who did not reach the predicted 6MWT values were 64%, 75%, and 100% for radiographic lesion scores of grades I, II, and III, respectively. There were no important changes in the systemic blood pressure, heart rate, SpO2, and Borg scale values during the tests. All of the subjects had a normal nutritional status (see Table 2), and 78% reported an impairment in quality of life (Fig. 3). The Airways Questionnaire 20 scores ranged from zero to 90% (mean = 50.3%, 95% CI = 42.2–72.8%).
Characteristics of the Subjects (n = 18)
FEV1/FVC of the 18 pulmonary multiple-drug-resistance tuberculosis subjects.
Functional Measurements
Maximal inspiratory and expiratory pressure of the 18 pulmonary multiple-drug-resistance tuberculosis subjects.
Airways Questionnaire 20 scores of the 18 pulmonary multiple-drug-resistance tuberculosis subjects. Scoring range from zero (no disability) to 100%.
Discussion
The main factors that may affect the pulmonary function of patients who are treated for TB are the lack of adherence to drug therapy, residual post-treatment lesions, treatment duration, and the presence of other pulmonary diseases such as emphysema, COPD, asthma, and bronchiectasis. A lack of adherence was present among our subjects who developed MDR-TB, suggesting that treatment irregularity and abandonment are important factors that contribute to multiple-drug-resistance.14–16
In spite of a number of respiratory function reports, there is no consensus concerning the predominant type of ventilatory disorder found in patients treated for TB.14,17–20 Ramos et al17 found a homogeneous distribution in spirometric patterns (34% showed a mixed ventilatory disorder, 24% showed an obstructive disorder, 24% exhibited normal function, and 18% exhibited a restrictive disorder), while Pasipanodya et al18 reported that the restrictive pattern was the most frequent (31%). Cruz et al19 found a homogeneous distribution in normal function and restrictive ventilatory pattern. In contrast to the data provided by these authors, our results are in agreement with Maguire et al,20 who also diagnosed the obstructive pattern most frequently (39%).
Because smoking is an important risk factor for TB,21 and many of the physical and social factors that are associated with cigarette smoking are shared with TB,22 most of the studies addressing the functional aspects of these patients have included smokers. Moreover, Singla et al23 evaluated 63 patients with MDR-TB and did not find a difference between the pulmonary function of smokers and non-smokers. Our study confirmed these results. Consistent with the methods of these authors, we considered smoking to be a common finding in TB patients, and we did not exclude the subjects who had a history of smoking, because we wanted to preserve the external validity of the study. Moreover, because we did not detect an association between smoking and FEV1 or a statistically significant difference in the FEV1 values from smokers and non-smokers, it seems that a history of smoking was not a determining factor of the functional impairments observed in our study.
The large range of normality for maximal respiratory pressures makes the clinical interpretation of this outcome difficult; however, because these measurements are reproducible and our results were below the predicted values for all patients, we believe that some degree of respiratory muscle weakness is a common finding in MDR-TB patients. This finding is corroborated by the recent study performed by Di Naso et al,24 in which MDR-TB patients presented with low PImax and PEmax values, as in our study (49.58 ± 12.55 cm H2O and 59.08 ± 12.23 cm H2O, respectively). Because our subjects had normal body mass indexes and lean body mass (probably because they were in the MDR-TB post-cure stage, and there were no HIV patients in our sample25), the muscle weakness that we observed cannot be explained in terms of the impaired nutritional status commonly found in TB patients.26 In addition, it is well known that chronic respiratory diseases can affect the function of respiratory muscles.27,28 In this way, 78% of our sample had a ventilatory disturbance (obstructive, restrictive, or mixed), which represents a mechanical overload for the respiratory muscles and results in low PImax and PEmax values.28,29
All of these ventilatory impairments impact the functionality of TB patients. In this study, functionality was assessed using the 6MWT because it reflects the patients' performance of daily activities.30 Although we followed the ATS recommendations for the test, the large CI and the low determination coefficient of the Enright and Sherrill reference equation10 are limiting factors in the clinical interpretation of our 6MWT results. Nevertheless, many different equations with similar limitations have been proposed and used to interpret the 6MWT results in a number of studies. There was most likely no association between the respiratory maximal pressures and the 6MWT because exercise capacity depends on several factors.
Although several authors have reported a reduction in the functional capacity of patients with TB sequelae,31,32 we only found one other study evaluating the 6MWT of MDR-TB patients. As in our study, Singla et al23 found reduced 6MWT values in all 47 subjects studied. These respiratory function and exercise tolerance impairments are reflected in the quality of life for MDR-TB patients, but studies on this topic are scarce. Ramos et al17 have already recommended the early treatment of TB cases, which contributes to a decrease in sequelae and improves the patients' quality of life. Dhuria et al33 reported the impact that TB has on patients' quality of life, showing scores that were significantly lower than a control group. The authors reported that the main impaired domains were the physical domain, which involves the ability to perform daily activities, followed by the psychological domain. The systematic review developed by Guo et al34 also demonstrated that TB has a substantial negative impact on the patients' quality of life, even after the microbiological cure. The authors emphasize that a number of instruments were used to assess the quality of life in TB, but that none of them was specific, making it difficult to understand the impact of the disease in such a group of patients.
In our study, quality of life was assessed by the Airways Questionnaire 20, which is generally used for airway and respiratory diseases.34,35 The advantages of this questionnaire include a shorter assessment time, high reproducibility, and an excellent correlation with the Saint George's Respiratory Questionnaire.35 Because 78% of the subjects reported an impairment in their quality of life, our study is consistent with the literature, which states that TB patients have radiographically identifiable pulmonary sequelae even after healing, which jeopardizes lung function and quality of life.17–19,33,34
The main limitations of the study are the small sample size and the fact that some subjects in our sample were former smokers. In addition, it is unclear whether the functional impairments preceded TB or MDR-TB. Despite these limitations, this is the first study to evaluate the post-treatment sequelae in a cohort of Brazilian MDR-TB patients. Because there are few published studies regarding the functional parameters of this group of subjects, we believe that our results bring an important contribution to the field. Additional studies that describe the dysfunctions afflicting these patients and that compare pulmonary function before and after MDR-TB are necessary.
Conclusions
In conclusion MDR-TB cured patients present impaired respiratory function and a mildly reduced functional capacity and quality of life, suggesting that a portion of these patients may require a pulmonary rehabilitation approach.
Footnotes
- Correspondence: Sara LS Menezes PT PhD, Hospital Universitário Clementino Fraga Filho, Rua Professor Rodolpho Paulo Rocco, 255 - 8° Andar, 21941–913, Rio de Janeiro, Brazil. E-mail: menezes{at}hucff.ufrj.br.
The authors have disclosed no conflicts of interest.
- Copyright © 2012 by Daedalus Enterprises Inc.