Abstract
Choke points and airway wall structure in expiratory central airway collapse are poorly defined. Computed tomography, white light bronchoscopy, endobronchial ultrasound, vibration response imaging, spirometry, impulse oscillometry, negative expiratory pressure, and intraluminal catheter airway pressure measurements were used in a patient with cough, dyspnea, and recurrent pulmonary infections. Computed tomography and white light bronchoscopy identified dynamic collapse of the trachea and mainstem bronchi, consistent with severe crescent tracheobronchomalacia. Spirometry showed severe obstruction. Endobronchial ultrasound revealed collapse of the airway cartilage, and vibration response imaging revealed fluttering at both lung zones. Impulse oscillometry and negative expiratory pressure suggested tidal expiratory flow limitation in the intrathoracic airways. Intraluminal catheter airway pressure measurements identified the choke point in the lower trachea. After Y-stent insertion, the choke point migrated distally. Imaging studies revealed improved airway dynamics, airway patency, and ventilatory function. Novel imaging and physiologic assessments could be used to localize choke points and airway wall structure in tracheobronchomalacia.
- tracheobronchomalacia
- excessive dynamic airway collapse
- choke points
- air flow dynamics
- endobronchial ultrasound
- vibration response imaging
- impulse oscillometry
- airway stent
- rigid bronchoscopy
Introduction
Wave speed theory suggests that airway flow limitation occurs when flow velocity equals the speed of propagation of pressure-pulse waves at some point within the airway.1 This flow-limiting segment, called the choke point, tends to be located in a region of minimum cross-sectional area and minimum side pressure within the airway when maximal flow is reached. Because maximum expiratory flow is airway compliance-dependent, increased compliance, such as seen in patients with tracheobronchomalacia, results in increased airway resistance and decreased maximum expiratory flow. Results from studies of air-flow limitation in theoretical and experimental models demonstrate that when the collapsing trachea is supported by a rigid tube, air flow improves and the flow-limiting segment migrates from the central airway toward the periphery.2
Malacia, defined as weakness of central airway cartilaginous structures, is a form of expiratory central airway collapse. Another form of this syndrome, however, excessive dynamic airway collapse, is defined as excessive inward bulging of the posterior membrane in the absence of weakened cartilage. Excessive dynamic airway collapse can be erroneously described as malacia on imaging studies and bronchoscopy.3 While both are forms of expiratory central airway collapse, choke points in patients with excessive dynamic airway collapse are probably located peripherally, while in patients with malacia they appear to be located centrally.3
Based on the above-mentioned concepts, procedures targeting central airway stabilization, such as surgical tracheobronchoplasty, bronchoscopic stent insertion, and noninvasive positive-pressure ventilation, are offered to improve expiratory flow by increasing cross-sectional area, and support the weakened airway wall structures in selected patients with expiratory central airway collapse.3 Because selecting the appropriate airway stabilization procedure is not straightforward, various strategies and algorithms, including the use of stent insertion trials, have been proposed in the quest to optimize patient management.3,4 In this paper we describe how novel multimodality imaging studies and physiologic assessments might be combined in order to assess airway wall structure, identify changes in flow-limiting segments before and after treatment, and provide further insights into the pathogenesis of expiratory central airway collapse.
Case Report
A 68-year-old man presented with dyspnea on exertion, heavy sputum production, and chronic cough. His medical history consisted of chronic bronchitis and tuberculosis. Physical examination revealed bilateral rhonchi and early wheezing on expiration. He had grade IV on the Medical Research Council dyspnea scale. Laboratory findings showed a white blood cell count of 13,200 cells/μL and C-reactive protein of 1.06 mg/dL, due to chronic inflammation. Sputum cultures on repeated occasions showed Pseudomonas aeruginosa and Achromobacter xylosoxidans. Computed tomography (CT); white light bronchoscopy; endobronchial ultrasound; vibration response imaging; and physiologic assessments including spirometry, impulse oscillometry, negative expiratory pressure, and intraluminal catheter airway pressure measurements were applied to determine how novel imaging and physiologic technologies can be used to elucidate choke point physiology and airway wall structure in the setting of expiratory central airway collapse. The study was approved by the St Marianna University internal review board, and the patient provided informed consent.
Spirometry revealed an FVC of 2.27 L, FEV1 of 0.74 L, and peak expiratory flow (PEF) of 2.48 L/s. The flow-volume curve showed marked reduction of the expiratory flow (Fig. 1). Paired inspiratory and expiratory dynamic CT findings showed tracheal and bilateral mainstem bronchial collapse during the expiratory phase (see Fig. 1). White light bronchoscopy confirmed the radiographic findings, suggesting severe diffuse crescent type tracheobronchomalacia and airway wall edema from chronic inflammation (Fig. 2).
A: Before stent insertion, spirometry (left panel) shows FVC of 2.27 L, FEV1 of 0.74 L, peak expiratory flow (PEF) of 2.48 L/s, and an airway collapse pattern. Impulse oscillometry (middle panel) shows R5 of 0.92 kPa/L/s (inspiratory 0.76 kPa/L/s, expiratory 1.05 kPa/L/s), R20 of 0.51 kPa/L/s (inspiratory 0.42 kPa/L/s, expiratory 0.59 kPa/L/s), X5 of −0.57 kPa/L/s (inspiratory −0.37 kPa/L/s, expiratory −0.81 kPa/L/s), frequency of resonance of 24.63 L/s (inspiratory 21.77 L/s, expiratory 26.92 L/s). Negative expiratory pressure (right panel) shows the tidal expiratory flow limitation. B: Twelve days after stent insertion, spirometry shows FVC of 2.23 L, FEV1 of 0.98 L, PEF of 2.99 L/s. Impulse oscillometry reveals R5 of 0.48 kPa/L/s (inspiratory 0.49 kPa/L/s, expiratory 0.48 kPa/L/s), R20 of 0.26 kPa/L/s (inspiratory 0.24 kPa/L/s, expiratory 0.28 kPa/L/s), X5 of −0.25 kPa/L/s (inspiratory −0.2 kPa/L/s, expiratory −0.28 kPa/L/s), frequency of resonance of 20.29 L/s (inspiratory 20.79 L/s, expiratory 19.37 L/s). Negative expiratory pressure shows no tidal expiratory flow limitation. Paired inspiratory (C) and expiratory (D) dynamic computed tomography reveals lower tracheal collapse during expiration.
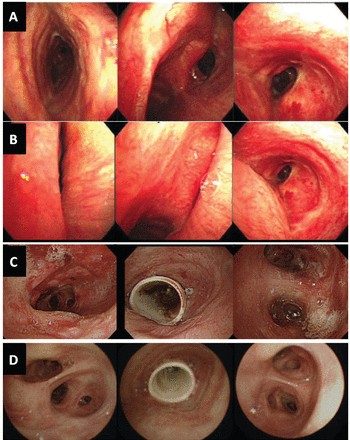
Bronchoscopy images obtained during inspiration (A) and expiration (B) before stent insertion. Left: left main bronchus. Middle: carina. Right: trachea. Tracheobronchial lumen images during bronchoscopy shows the trachea, right main, and left main bronchi collapsed during the expiratory phase. C: One day after stent insertion, airway patency has improved, but there is substantial mucosal erythema and edema. Left: bronchus intermedius. Middle: trachea. Right: distal left main bronchus. D: Thirty days after stent placement, chronic airway inflammation has improved.
In order to further elucidate airway wall structures and flow-limiting segments, additional imaging and physiologic studies were performed (Table 1). Endobronchial ultrasound, using a 20 MHz radial probe (UM-3R, Olympus, Tokyo, Japan) and a flexible sheath equipped with a balloon at the tip (MH-246R, Olympus, Tokyo, Japan) revealed expiratory collapse (horizontalization) of the hyper-echogenic layer corresponding to the weakened airway wall cartilage, confirming the diagnosis of malacia (Fig. 3). The sub-mucosal layer was thick due to chronic inflammation. The vibration response imaging system (VRI, Deep Breeze, Or-Akiva, Israel) showed a floating image and fluttering at both lungs, representative of central airway turbulence (see Fig. 3). Impulse oscillometry (Masterscreen, Jaeger, Höchberg, Germany) revealed resistance at 5 Hz (R5) of 0.92 kPa/L/s, resistance at 20 Hz (R20) of 0.51 kPa/L/s, R5–R20 of 0.41 kPa/L/s, reactance at 5 Hz (X5) of −0.57 kPa/L/s, frequency of resonance 24.63 L/s (see Fig. 1). These results of impulse oscillometry showed a marked frequency dependence of the respiratory system resistance, namely R20 substantially decreased from R5. The elevated R5 represents the narrowing of central airways, including the trachea and main bronchi. The decreased R20 signifies that the higher frequency component of the pressure pulse could not pass through the narrow central airway to the distal airways, but resulted in sequential inflation and deflation of the collapsible airway segments. Impulse oscillometry data also showed the marked difference of X5 between inspiratory and expiratory phases. The significant decrease of X5 in the expiratory phase exhibits the tidal expiratory flow limitation.5 Since the choke point (ie, the flow-limiting segment) was established in the central airway during tidal expiration, the pressure pulse, especially the lower frequency component of 5 Hz, could not pass through the choke point to the distal airways. Therefore, the reactance component of 5 Hz represents the compliance of airway localized downstream of the choke point, and results in more negative X5. In contrast, since the choke point does not exist during inspiration, X5 represents the compliance of a broader range of bronchi, lung parenchyma, and chest wall. This resulted in less negative X5 and marked difference in measured values between the respiratory phases. Negative expiratory pressure measurements (HI-801, Chest MI, Tokyo, Japan) showed complete expiratory flow limitation (see Fig. 1).
Imaging and Physiologic Studies Before and After Stent Insertion
Endobronchial ultrasound shows (A) trachea, and (B) right main bronchus. Tracheal and bronchial cartilages were intact, but the tracheal cartilage was flat (horizontal) during expiration, suggesting weakness. The submucosal layer of the trachea and right main bronchus was thick (1) due to inflammation, and the tracheal cartilage shows a slight thickening with normal bronchial cartilage (2). C: Vibration response imaging shows a floating image (1) and fluttering image (2) in both lungs before stent insertion. D. After stent insertion, floating and fluttering disappeared.
The patient underwent rigid bronchoscopy under general anesthesia. Airway pressures were measured intraoperatively using an intraluminal pressure catheter (Fuji Systems, Tokyo, Japan) (Fig. 4). Large pressure differences were seen between the upper trachea and the carina, consistent with a location of the choke point in the lower trachea (see Fig. 4). A Y-shaped silicone stent (16.60 × 13.10 × 13.45 mm) was then inserted at the main carina (TM stent, Fuji Systems, Tokyo, Japan). Intraoperative airway pressures measured immediately after stent insertion showed that the pressure differences had disappeared (see Fig. 4). Results from subsequent follow-up studies post-intervention showed only slight improvement in spirometric values and flow-volume loop, with a substantial decrease in R5 on impulse oscillometry, and no expiratory flow limitation on negative expiratory pressure (see Fig. 1). Dyspnea, however, had improved to Medical Research Council grade II, and vibration response imaging showed that the floating and fluttering had disappeared (see Fig. 3). Flexible bronchoscopy confirmed the patency of the indwelling silicone stent and showed improvement of inflammatory mucosal changes (see Fig. 2).
A: A 3-dimensional bronchial tree image using Pulmonary Workstation 2 (Vida Diagnostics, Coralville, Iowa) with lateral pressure measurements. B: Before stent insertion a large pressure difference was seen between the upper trachea and right lower bronchus and carina. C: After stent insertion the pressure difference disappeared both during inspiration and expiration, and a regular respiratory cycle was seen.
Discussion
Tracheobronchomalacia and excessive dynamic airway collapse are both forms of expiratory central airway collapse, manifested by a similar constellation of symptoms.3,4 The 2 disorders are different from each other even though in the literature the term “malacia” is often used to describe excessive dynamic airway collapse.3 Determining ideal treatment might prove difficult in selected cases, prompting therapeutic trials to determine ideal outcomes.3,4 In this report, multimodality imaging and physiologic studies were combined to provide additional insight into the pathophysiology of tracheobronchomalacia.
Paired inspiratory-expiratory dynamic CT scanning can reveal the degree and potentially the etiology of central airway collapse.6 Disagreement exists, however, regarding how much of a decrease in cross-sectional area signals clinically and physiologically important airway narrowing.3,7 While many investigators use 50% or more reduction in airway cross-sectional area between inspiration and expiration to identify malacia,3 this definition leads to over-diagnosis, considering that 78% of normal individuals reportedly exceed this criterion.7
Endobronchial ultrasound using a 20 MHz radial probe identifies hypo- and hyper-echoic layers that correlate with the laminar histological structures of the central airways.8 Structural airway wall abnormalities have been identified in patients with malacia caused by tuberculosis, relapsing polychondritis, lung cancer, compression by vascular rings, and in patients with excessive dynamic airway collapse.8–10 Furthermore, endobronchial ultrasound could potentially distinguish between tracheobronchomalacia and excessive dynamic airway collapse. In tracheobronchomalacia, for example, the cartilage is destroyed or weakened,8–10 while in excessive dynamic airway collapse it appears that the cartilage is intact and the posterior membrane is thinner than normal, likely due to atrophy of elastic fibers.10
Vibration response imaging is a noninvasive imaging tool that can be described as an electronic stethoscope that picks up the sounds from the chest, using 40 piezo-acoustic sensors. Analog signals are transformed into dynamic gray-scale images, similar to the process involved in ultrasound imaging.11 Vibration response imaging has been used in the evaluation of patients with asthma, COPD, aspiration of foreign objects, and tracheobronchial obstruction undergoing bronchoscopic interventions.11 To our knowledge, however, this is the first report of vibration response imaging findings in a patient with expiratory central airway collapse. The disappearance of floating and fluttering post-stent insertion is consistent with previous studies showing improvement in patients with other forms of central airway obstruction after bronchoscopic interventions.11 Experimental studies suggest that sounds at frequencies of 100–250 Hz are mainly generated in the central airways, 250–350 Hz in the smaller bronchi, 500–650 Hz in the terminal bronchioles, and 650–1,500 Hz within the alveolar space.12 Thus, differential analysis of vibration response imaging might allow precise localization of pathologic processes in different compartments of the lung.11,12 In this regard, tracheobronchomalacia and excessive dynamic airway collapse should provide different dynamic gray-scale images, since in tracheobronchomalacia the flow-limiting segments are predominantly central, while in excessive dynamic airway collapse they are peripheral.
Spirometry in patients with expiratory central airway collapse may reveal obstructive ventilatory impairment proportionate to the severity of the disease, but these findings are nonspecific.3 The descending expiratory limb might reveal a peak and plateau in the flow-volume loop (the so called “airway collapse” pattern), which, while indicative of central airway collapse, is neither sensitive nor specific for malacia.3 Furthermore, spirometry measurements after treatment are not necessarily representative of the degree of symptomatic improvement, as demonstrated in our patient.13 This observation is similar to findings in patients with COPD, in whom therapy with bronchodilators can improve dyspnea and exercise endurance, with little or no associated change in maximal expiratory flow rates, despite evidence of improvement in physiologic measurements of dynamic hyperinflation.14 Therefore, treatment by stent insertion, which stabilizes and reduces the central airway flow turbulence, might also result in clinical improvements that are not necessarily detectable using FEV1 measurements.14
Contrary to spirometry, negative expiratory pressure measurements do not require patient collaboration, performance of FVC maneuvers, or use of a body plethysmograph. Negative expiratory pressure can be used, apart from in spontaneously breathing subjects in any body position, during exercise, and in the intensive care unit setting.5 Negative expiratory pressure identifies expiratory flow limitation by comparing the expiratory flow-volume profile of a tidal breath (ie, control breath) to that of a breath when additional negative pressure is applied (ie, test breath).5,15 The term expiratory flow limitation is used to indicate that maximal expiratory flow is achieved during tidal breathing and is characteristic of intrathoracic air-flow obstruction. Tests are performed by applying negative pressure (−3 to −5 cm H2O) at the mouth during a resting tidal expiration and comparing the ensuing expiratory flow-volume loop with that of a previous control tidal expiration. The physiologic premise for negative expiratory pressure measurements is that any increase in flow beyond that obtained from the control breath demonstrates that some expiratory flow reserve is present. If negative expiratory pressure elicits increased flow over the entire control tidal volume, the subject is not flow limited. In contrast, if with negative expiratory pressure the subject exhales partly or entirely along the control flow-volume curve, intrathoracic flow limitation is present. This method is independent of volume and time history, is noninvasive, and is readily applicable in a variety of clinical settings.5,15 However, negative expiratory pressure detects flow limitation in any condition during which all possible pathways between airway opening and alveoli are choked.16 In our patient, however, upper airway collapse during negative expiratory pressure test was implausible since he did not have clinical signs or symptoms of obstructive sleep apnea, and negative expiratory pressure measurements showed complete expiratory flow limitation before stent insertion and no expiratory flow limitation after stent insertion.
Impulse oscillometry is also an effort independent test during which brief random pressure pulses of 5–35 Hz, generated by a small loudspeaker mounted in series with a pneumotachograph, are applied during tidal breathing. Pressure-flow oscillations are superimposed on the subject's tidal breaths, and real-time recordings are used to provide an estimate of total respiratory system impedance, including measurements of resistance and reactance at different frequencies that might differentiate between central and peripheral components of airway obstruction.15 Increased resistance at a low oscillation frequency (5 Hz) reflects an increase in total respiratory resistance, suggestive of airway obstruction such as that found in patients with COPD, while an increase at a higher frequency (20 Hz) reflects more specifically increased central airway resistance such as that found in patients with malacia.17 The impulse oscillometry maneuver does not cause respiratory fatigue15 and may be more sensitive than spirometry. In this report we showed how impulse oscillometry measurements can identify narrowing and localize choke points in the central airways. Impulse oscillometry data from variable central airway obstruction are similar to those from patients with COPD (ie, increased resistance at 5 Hz, marked frequency dependence in resistance, more negative reactance at 5 Hz, and increased resonant frequency). Furthermore, the data were similar to those in severe COPD patients with tidal expiratory flow limitation (ie, reactance at 5 Hz was much more negative in the expiratory phase, compared with the inspiratory phase). In our patient the impulse oscillometry data normalized after stent insertion, confirming that the impulse oscillometry pattern was due to the central airway collapse. Since the differentiation of peripheral from central airway resistance is based on the existence of airway compliance in impulse oscillometry measurement in COPD patients,18 the increased collapsibility in the central airway in this patient was considered to be responsible for the similar results. Although the results of impulse oscillometry were like those of patients with COPD, R20 in patients with central airway obstruction is probably higher than in COPD, but this will need to be confirmed in future studies. Since in excessive dynamic airway collapse the predominant site of flow limitation is in the periphery, higher R5 and R5-R20 values are expected than with tracheobronchomalacia, for which the main site for flow limitation is the central airways, and thus higher R20 is expected.
In pondering the clinical relevance of the multiple modalities described in our study, we suggest that traditional investigations would have included spirometry, dynamic CT, and bronchoscopy, which would have demonstrated obstructive ventilatory impairment and expiratory central airway collapse most likely caused by tracheobronchomalacia.3,4,6,7 The addition of negative expiratory pressure and impulse oscillometry confirmed the diagnosis of obstructive ventilatory impairment and localized the expiratory flow limitation to the central airways. Endobronchial ultrasound confirmed malacia as the cause of expiratory central airway collapse, since the posterior membrane was normal but there was evidence of cartilage weakness and cartilage collapse during expiration. Intra-operative pressure measurements revealed the precise location of the flow-limiting segments in the central airways. After stent insertion, intra-operative pressure measurements revealed resolution of flow-limitation at previously identified central airway choke point locations, also demonstrated noninvasively by negative expiratory pressure and impulse oscillometry. Vibration response imaging revealed changes in display of the gray-scale images during the expiratory phase of the cycle post-intervention, suggesting a change in the anatomical location of the sites responsible for the generation of vibrations. Correlations of this Vibration response imaging pattern with choke point location, however, requires further investigations before firm conclusions can be drawn.
A clear understanding of choke point location in malacia may be important because of differences in comparison with excessive dynamic airway collapse, another form of expiratory central airway collapse that is not uncommon in patients with COPD, symptomatic asthma, and obesity.19,20 Excessive dynamic airway collapse is seen in patients with various forms of small airway obstruction in the absence of structural central airway abnormalities.3 Narrowing of the central airways is exaggerated in patients with small airway disease, in part because of loss of intraluminal pressure in the periphery (friction losses in small airways). The resulting negative transmural pressure gradient causes excessive invagination of the posterior wall. In these situations, pressure catheter measurements demonstrate no pressure drop along the trachea and mainstem bronchi, thus predicting no improvement in air flow after central airway stabilization procedures.21 It is therefore expected that pressure catheter measurements in excessive dynamic airway collapse reveal no pressure drop along the collapsible central airway, contrary to tracheobronchomalacia, as revealed in this report. While a major limitation of our study is the absence of direct comparison with a patient with excessive dynamic airway collapse, we submit that studies are warranted to determine whether multimodality imaging and physiologic assessments might help distinguish malacia from excessive dynamic airway collapse, and to precisely localize choke points in both disorders, thereby providing information to assist in proper patient selection for conservative, minimally invasive, or open surgical procedures to stabilize the central airways.
In summary, a combination of novel diagnostic imaging and physiologic assessment technologies were applied to elucidate the characteristics of choke point physiology and airway wall structure in a patient with severe diffuse crescent-type malacia. The central airway location of flow-limiting segments was precisely localized, and choke point analysis was concordant with physiologic models predicted by wave-speed theory of expiratory flow limitation. Airway stabilization at the choke point improved flow limitation by increasing the airway cross-sectional area and supporting the weakened airway wall, thus improving ventilatory function and relieving dyspnea.
Footnotes
- Correspondence: Septimiu D Murgu MD, Pulmonary and Critical Care Medicine, University of California School of Medicine, 101 The City Drive, South City Tower, Suite 400, Orange CA 92868-3217. E-mail: smurgu{at}uci.edu.
The authors have disclosed no conflicts of interest.
- Copyright © 2012 by Daedalus Enterprises Inc.