Abstract
Mechanical ventilation is a life-saving supportive therapy, but it can also cause lung injury, diaphragmatic dysfunction, and lung infection. Ventilator liberation should be attempted as soon as clinically indicated, to minimize morbidity and mortality. The most effective method of liberation follows a systematic approach that includes a daily assessment of weaning readiness, in conjunction with interruption of sedation infusions and spontaneous breathing trials. Protocols and checklists are decision support tools that help ensure consistent application of key elements of evidence-based practice. A majority of studies of weaning protocols applied by non-physician healthcare providers suggest faster weaning and shorter duration of ventilation and ICU stay, and some suggest reduced failed extubation and ventilator-associated pneumonia rates. Checklists can be used to reinforce application of the protocol, or possibly in lieu of one, particularly in environments where the caregiver-to-patient ratio is high and clinicians are well versed in and dedicated to applying evidence-based care. There is support for integrating best-evidence rules for weaning into the mechanical ventilator so that a substantial portion of the weaning process can be automated, which may be most effective in environments with low caregiver-to-patient ratios or those in which it is challenging to consistently apply evidence-based care. This paper reviews evidence for ventilator liberation protocols and discusses issues of implementation and ongoing monitoring.
Introduction
Mechanical ventilation is a common life support modality in ICUs. The process of ventilatory support follows a continuum of care, beginning with the patient requiring initial support and hopefully ending with the ability to sustain spontaneous breathing (Fig. 1). Some patients move through the process quickly, while others require a longer period, and some do not make it through at all. Throughout the process, many patient assessments are made and ventilator adjustments executed to accomplish the therapeutic goals of improving oxygenation and ventilation, increasing patient comfort, and minimizing the likelihood of causing secondary injury such as ventilator-induced lung injury, ventilator-associated diaphragmatic dysfunction, or ventilator-associated pneumonia (VAP).
Continuum of care of the mechanically ventilated patient.
The focus of this review is the final phase of the continuum: the process of ventilator discontinuation or weaning. The term weaning historically implied a gradual reduction in ventilator support, to allow patients the ability to assume increasing levels of work to breathe, and was often drawn out over several days or longer.1 Current evidence consistently has shown that when issues causing respiratory failure are adequately addressed and the patient is ready to be liberated from the ventilator, this process can be done quickly. Some authorities emphasize a distinction between weaning versus ventilator liberation or ventilator discontinuation,2,3 because these terms imply a quick process and help to change the clinician's mindset. The phrase “to wean” can also mean to stop, to terminate, or to halt, and the concept of a liberation process or discontinuation process by itself does not necessarily imply a quick or short duration. The key point is not which term to use but rather to understand that when the ventilator is no longer needed, it should be stopped as soon as clinically possible, which for most patients is an immediate or abrupt step. Regardless of what we call the process, a gradual reduction in support is not appropriate for most patients.
The specific goals of this discussion are to describe the evidence supporting ventilator discontinuation protocols and to describe elements required for successful implementation of such protocols.
Background
As disease acuity and therapeutic sophistication continue to escalate, the management of critically ill patients becomes more complex and the possibility of suboptimal care increases. Suboptimal care can take the form of medical errors,4 as well as failure to implement evidence-based practice.5,6 Guidelines, checklists, and protocols are decision support tools used to reduce practice variation and instill evidence-based practice into clinical care.5,7–10 Guidelines are general statements that lack specific detail and provide broad guidance, allowing clinicians to use their own judgment and experience to fill in gaps in the instructions. They allow different decisions to be made by different clinicians for the same clinical scenario.7 Protocols, on the other hand are, considered “adequately explicit” in that they contain enough detail that different clinicians will arrive at the same decision for the same clinical scenario.7 They are often referred to as algorithms. Checklists contain a list of action items or criteria arranged in a systematic order to discuss during patient rounds.11 They help to ensure that all elements of critical patient care processes are consistently considered.
Protocols include the important “rules” that an expert clinician would use to make clinical decisions, and allow less experienced clinicians to arrive at the same decision. In theory, this allows each patient to receive the highest level of care, independent of the skill level of the clinician providing the care. Critics decry this as a “cookbook approach” to medicine that takes away clinician decision making ability and minimizes the development of critical thinking skills.12 Proponents argue that protocols are tools that complement and enhance the clinical decision makers—not replace them. Protocols may actually enhance clinical teaching and practice if the protocols are dynamic and updated with evidenced-based rules, and if clinicians are able to describe the rationale and evidence for the rules.13
Research suggests that the use of protocols to guide the ventilator discontinuation process may improve outcomes. Although the number of hospitals using weaning protocols is unclear, a recent report suggests they are widely available in teaching hospitals. Prasad et al14 surveyed all adult ICU medical directors in United States teaching hospitals on the availability of protocols in 5 clinical areas, including ventilator weaning. Ventilator liberation was the most common protocol available in the ICUs, identified to be present in 77 of the 90 (89%) reporting hospitals. A majority (65%) had implemented the protocol for > 3 years, while 27% had done so within 1–3 years, and the reminder (8%) within a year. In 67% of the weaning protocols, the protocol was initiated either automatically (27%) or by a respiratory therapist (40%) without any physician interaction required. The respiratory therapist was identified as the primary driver of the protocol in 90% of the ICUs. Interestingly, the higher teaching intensity hospitals (resident-to-bed ratio > 0.6) were more inclined to have a weaning protocol than those with a lower ratio. Although protocols appear to be widely available, this study did not evaluate protocol compliance or the impact of the protocol on clinical outcomes.
Evidence for Protocols
The concept of weaning protocols, particularly those managed by non-physician healthcare providers, became popularized with the 2001 publication of the results of a task force on ventilator discontinuation.15 This project, facilitated by the American College of Chest Physicians, the American Association for Respiratory Care, and the American College of Critical Care Medicine, reported 12 evidence-based recommendations (Table 1) related to the ventilator discontinuation process. Each recommendation was given a letter grade denoting the level of evidence supporting it. The 3 levels used were: Grade A (evidence supported by well conducted, controlled trials with statistically significant results consistently supporting the recommendation), Grade B (evidence supported by observational studies or by controlled studies with less consistent results to support the recommendation), and Grade C (expert opinion supported the recommendation but scientific evidence either provided inconsistent results or was lacking). The recommendation that weaning protocols, executed by non-physician healthcare providers (recommendation 8 in the table), be used because they can reduce the duration of mechanical ventilation and decrease associated cost, was considered to have Grade A evidence. At the time of the task force's review, there were 3 randomized controlled trials (RCTs) totaling 1,042 patients.16–18
Evidence-Based Guidelines for Weaning and Discontinuing Ventilatory Support
The first report, by Ely and colleagues,16 studied 300 adults in medical and coronary ICUs. The intervention (protocol) group underwent daily screening of respiratory function by respiratory therapists and nurses to identify patients ready to undergo a 2 hour spontaneous breathing trial (SBT). Physicians were notified of patients passing the SBT. The control group received a daily screen but no other intervention. Study results suggested a decreased duration of ventilation, lower costs, and fewer reintubations in the protocol group.
The second report, by Kollef and colleagues,17 studied 357 adults in medical and surgical ICUs at 2 hospitals. The intervention group received daily screening for weaning readiness, followed by protocolized weaning using either SBTs, pressure support ventilation (PSV), or synchronized intermittent mandatory ventilation (SIMV). Weaning in the control group was physician-directed. Results of this study showed decreased duration of ventilation and hospital costs associated with the protocol group.
The third report, by Marelich and colleagues,18 studied 335 adults in medical and surgical ICUs. Patients in the intervention group were screened twice daily for weaning readiness and, if passing, were subjected to a 30 min SBT using either flow-by, PSV, or a T-piece. Control patients received standard ICU care, including physician-directed weaning. In addition to a decreased duration of ventilation, protocolized weaning was associated with a trend for a reduced rate of VAP in a subset of trauma patients.
Since these early reports other studies have provided mixed results. Krishnan and colleagues19 studied 299 adults who were mechanically ventilated for more than 24 hours. The intervention group received daily screening for weaning readiness, followed by an SBT by either nurses or respiratory therapists, while the control group received usual care per physician direction. This report found no important differences between groups in any outcome and concluded that protocols are not necessary in a closed ICU with generous staffing and structured physician rounds. An accompanying editorial20 suggested, “the question is not what went wrong with protocolized weaning but what was right with usual care.” Usual care in this study was at a pretty high level. The hospital participated in the original ARDS Network study,21 which included protocolized ventilator management and weaning, for several years before beginning this study. The physicians used checklists during rounds, which included elements of best practice gleaned from recent weaning studies. The physicians were also better staffed (∼9.5 physician-hours/bed/d), compared to the earlier studies by Ely (3.5 hour),16 Kollef (4.0 hour),17 and Marelich (4.7 hour),18 which showed improved outcomes with protocols. It appears that the protocolized weaning process used by respiratory therapists and nurses in the Krishnan study performed at least as well as weaning guided by highly trained, well staffed physicians.
Navalesi and colleagues randomized 318 adults from a single center 9-bed closed neurologic ICU.22 Patients in the intervention arm were screened daily for weaning readiness and, if passing, underwent a 1-hour SBT. Control patients received routine care, which included physician-directed weaning. There was no difference in ICU mortality, rate of tracheostomy, duration of ventilation, or ICU stay. There was a lower incidence of failed extubation in the protocol (5%) group versus the control (12%) group. They also surveyed clinicians about their perceptions of using a protocol, including improved job satisfaction, and found more favorable scores with nurses and physiotherapists than with physicians.
The concept of assessing the patient's readiness to wean and then reducing support has been automated and introduced into mechanical ventilators. One such system has been evaluated in several randomized trials. Lellouche et al reported a multicenter RCT of 144 patients.23 The control group underwent physician-directed weaning, and the study group an automated gradual reduction of PSV followed by a period of spontaneous breathing and then an alert of a successful SBT. The computer-controlled weaning group was associated with a shorter median duration of weaning (3 d vs 5 d), duration of ventilation (7.5 d vs 12 d), and ICU stay (12 d vs 15.5 d), as well as a reduced incidence of noninvasive ventilation post-extubation (19% vs 37%), compared to the control group.
Rose and colleagues reported a single-center Australian study of 102 adults.24 The control group used a nurse-directed weaning process, and the intervention group used the automated SmartCare computer-driven weaning process. There were no significant differences in any outcome measure. As in the Krishnan study, the accompanying editorial25 suggested that the important question to ask is, “What was right about management of the usual care group rather than what went wrong with computer-driven weaning group?” Of note, the nurse-to-patient ratio was staffed to a 1:1 ratio and their ICU was staffed with intensivists who rounded twice daily. Compared to the Lellouche study, Rose included younger and less sick patients, fewer COPD and more trauma patients, and fewer medical patients, all of which are associated with shorter duration of ventilation. It appears that the automated system performed at least as well as a process staffed by experts who have adequate time to spend at the patient's bedside to make frequent ventilator adjustments.
Schadler and colleagues recently reported an RCT of 300 postoperative patients from 3 ICUs in a single center who were weaned using either daily SBTs or the SmartCare system.26 There was no difference in duration of ventilation between groups for the group as a whole, but there was a shorter duration of ventilation identified in a subset of postoperative cardiac patients who weaned using the automated system (24 h vs 35 h).
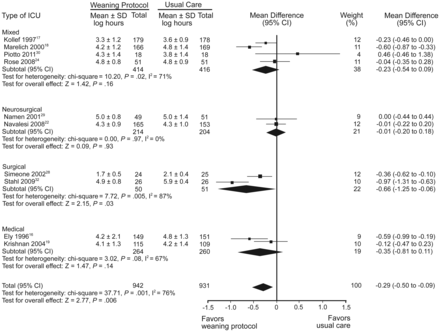
A recent meta-analysis of the weaning protocol literature by Blackwood and colleagues included 11 RCTs that totaled 1,971 patients.27 Eight studies compared a protocol driven by respiratory therapists or nurses versus physician-directed weaning,16–19,22,28–30 and 3 studies compared a computerized protocol versus physician-directed or standard care24,31,32 (Table 2). All but one of the clinician-driven protocol studies included a 2-step process of a daily screen for weaning readiness followed by SBT. Although weaning protocols were not associated with a difference in mortality, they were associated with a 25% reduction (P = .006) in the mean duration of mechanical ventilation, with the largest impact on surgical patients (48% reduction) (Fig. 2). Clinician led protocols were associated with a 22% reduction (P = .009) in duration of ventilation, whereas computer-driven protocols had a nonsignificant (P = .28) 39% reduction. This analysis did not include the Lellouche et al study,23 because the control physician-driven weaning arm used a protocol too.
Characteristics of Studies Included in the Blackwood27 Meta-analysis
Duration of mechanical ventilation with and without weaning protocol; subgroup analysis by type of unit. Mean difference calculated with fixed effects model (From Reference 27, with permission.)
Protocolized weaning is associated with hastening the weaning process and reducing total duration of mechanical ventilation in many institutions. However, protocols may not have as important an impact in settings where the standard of care already incorporates the evidence-based elements of weaning into everyday practice, particularly in environments where the clinician-to-patient ratio is high.
Identifying When to Start the Process
The process of ventilator liberation should begin as early as clinically possible. The 2001 American College of Chest Physicians guidelines15 suggest that a daily formal assessment should be made to determine the patient's readiness to wean. They recommend (see Table 2) using what some refer to as common-sense criteria2: evidence of reversal of the underlying cause for respiratory failure, adequate oxygenation on low PEEP, hemodynamic stability, and the ability to initiate an inspiratory effort.
Predicting Weaning Success
How do we know whether a patient meeting weaning readiness criteria will be liberated successfully? Historically, respiratory therapists obtain measurements of respiratory mechanics and lung volumes, such as VT, respiratory rate, minute ventilation, vital capacity, and maximal inspiratory pressure, prior to initiating the weaning process. Individually, most of these parameters do not have high accuracy in predicting weaning success or failure (Table 3) and one study recommended that they not be used.34 To improve accuracy, individual variables have been combined into integrated indices. The ratio of respiratory rate to VT (f/VT, the rapid-shallow breathing index) is the most widely used weaning parameter38 and often used in protocols. Although suggested to be one of the most predictive tests of successful weaning,37,39 f/VT has also been associated with prolonging the weaning duration when included in a weaning protocol, and suggested to not be routinely used in the weaning decision process.40 Three integrated weaning indexes of interest include the CROP index, the CORE index, and the Integrated Weaning Index (IWI) (Table 4 shows the formula of each). The CROP index takes into consideration the patient's compliance, respiratory rate, oxygenation (PaO2/PAO2), and maximal inspiratory pressure,35,37 while the CORE index uses the same elements and adds a measure of respiratory drive (airway-occlusion pressure 0.1 s after the start of inspiratory flow [P0.1]).35 The IWI uses compliance, SaO2, and f/VT, and does not include the patient dependent maximal inspiratory pressure.36 Table 3 shows the improved predictive performance for the CPOP index using a threshold higher than originally described, and the high performance level of the CORE index and IWI. Further research is required to determine the clinical value of these weaning indexes.
Performance of Various Weaning Indices as Predictors of Weaning Success
Formulas for 3 Integrative Weaning Indexes
Identifying an accurate predictor for patients likely to wean successfully remains elusive. Currently, the SBT has been identified as the main diagnostic test to determine whether patients are ready to be liberated from the ventilator.15,41
Support During the Spontaneous Breathing Trial
The ideal level of ventilatory support during an SBT is controversial. Studies report using a T-piece, CPAP of 5 cm H2O, PSV of ≤ 8 cm H2O (the level sometimes dependent on diameter of the artificial airway), CPAP with PSV, automatic tube compensation (ATC), and ATC with CPAP. Early studies suggested that SBTs done on T-piece, PSV of 5–7 cm H2O, or CPAP of 5 cm H2O ended in similar results and the methods essentially equivalent.42,43 Although early studies of ATC compared to PSV or T-piece suggested that ATC might facilitate a higher rate of successful SBTs,44,45 a recent comparison of ATC versus CPAP during the SBT showed no difference in the duration of weaning, duration of ventilation, or rate of extubation failure.46
A major goal of the SBT is to allow patients to demonstrate that they are ready to assume the work of breathing in preparation for removing the artificial airway. Ideally, clinicians want to observe the patient under conditions of respiratory load that would simulate those following extubation. The concept of providing minimal ventilator settings, such as PSV of 5–7 cm H2O or CPAP of 5 cm H2O, may be faulty on several levels.47
Assuming an artificial airway adds resistance that is not present when it is removed, PSV or ATC is often applied in an attempt to simulate breathing without the tube. Unfortunately, inflammation and edema develop in the upper airways after an endotracheal tube has been in place for several days. It has been shown experimentally that the work of breathing through an endotracheal tube, compared to the work of breathing following extubation, is almost identical.48 Providing any level of pressure support may cause clinicians to overestimate certain patients' capacity to sustain ventilation following extubation.49 It has been shown that PSV of 5 and 10 cm H2O can reduce inspiratory work by 31–38% and 46–60%, respectively.50,51
Similarly, in ventilated patients the addition of 5 cm H2O CPAP can reduce the work of breathing by 40%.51 PEEP or CPAP can also produce an increase in cardiac output in patients with left ventricular failure.52 Abrupt elimination of the positive pressure, as with extubation, can precipitate cardiopulmonary decompensation in these patients.53
Healthy biologic systems appear to have natural variability, and loss of variability is associated with disease or an unhealthy system.54 Reduced breathing pattern variability during weaning has been associated with weaning failure.55,56 Bien and colleagues studied this variability in 68 patients who underwent an SBT while breathing randomly under 3 conditions: T-piece, PSV of 5 cm H2O with 5 cm H2O PEEP, or ATC with 5 cm H2O PEEP.57 At the end of the 3 trials the patients were extubated. Extubation failure was associated with reduced breathing pattern variability. Although PSV was associated with less variability in the breathing pattern than was ATC or T-piece breathing, the successful extubation predictive value of f/VT measured during T-piece breathing was lost when measured during either ATC or PSV.
Because PSV, CPAP, and ATC can influence the breathing variability and hemodynamic response, some propose that the SBT be completed while providing no additional support.2 Although this can be done using a T-piece, current practice is generally to keep the patient attached to the ventilator for monitoring purposes, with PSV and PEEP set to zero to accomplish a similar effect.
For most patients it may not make a difference whether the SBT is done on T-piece, low level PSV, CPAP, or ATC, but in certain patients these low levels of support may give clinicians a false sense of security of the patient's capability. Therefore, a short period of unassisted breathing may be warranted before extubation.49
A New Weaning Classification and Clinical Implications
A recent international task force on weaning reiterated many of the recommendations made in 2001 and also recommended that patients be categorized into 3 weaning groups, based on the difficulty and duration of the weaning process.41 Simple weaning is defined as successful weaning and extubation after the first SBT; difficult weaning requires up to 3 SBTs or as long as 7 days to successfully wean; and prolonged weaning describes those failing more than 3 SBTs or requiring more than 7 days to be successfully liberated once the process begins.
The initial report41 suggested that the incidence of patients falling into the simple category was 69%, and another 15% in each of the other 2 categories. Mortality was reported to be 5%, 15%, and 15%, respectively. Several studies have applied this new classification and reported the incidence and ICU mortality for their patients (Table 5).58–61 These recent reports suggest that over time the incidence of those in the simple weaning category is declining and those in the difficult category increasing. This may be related to the screening criteria used to suggest weaning readiness. In the past it was recommended that acceptable oxygenation was sustained with FIO2 ≤ 0.40 and PEEP ≤ 5 cm H2O, while now it is common practice to use an FIO2 of ≤ 0.50 on PEEP of ≤ 5–8 cm H2O. It is not surprising that sicker patients may require more frequent weaning attempts. These recent reports also suggest that the mortality difference may not be different between those who pass the first SBT and those requiring up to 3 SBTs, and that the mortality rate significantly increases for patients requiring either > 3 SBTs or 7 days to be liberated from the ventilator.
New Weaning Classification Based on Duration of Liberation
The clinical implications of this classification suggest that for the simple weaning group the main objective is to identify a patient's readiness to wean as soon as possible, and this is best done with a systematic approach, such as via a protocol.41,62 For the difficult to wean patient, a major objective is identifying and addressing reversible causes for SBT failure, in which muscle weakness may be implicated. In the prolonged weaning patient, muscle weakness is most likely a major factor. Preventive measures such as encouraging spontaneous breathing or at least triggering of the ventilator, well controlled use of sedation, and early mobilization may help.63,64
Sedation and Weaning
Providing adequate sedation and analgesia is an important aspect of managing patients requiring ventilatory support. A goal is to provide levels that minimize pain and anxiety without interfering with the patient's ability to assume spontaneous respirations. The value of a daily interruption of sedation infusions, particularly on reducing the duration of ventilation and ICU stay, has been known for some time, and many institutions have developed sedation protocols.65–67 Recent evidence suggests that the sedation protocol and weaning protocol should be linked, in that the assessment of weaning readiness should be conducted during the spontaneous awakening trial of reduced sedation, to maximize the patient's breathing potential.68 This pairing of the spontaneous awakening trial and SBT should be the standard of care, as it may reduce mortality.69
Deciding to Extubate
After the patient demonstrates the ability to sustain spontaneous breathing, the next decision to make is whether the patient can tolerate extubation. It is an important decision, as both delayed extubation and failed extubation are associated with an increase in duration of ventilation and mortality. Several studies have evaluated risk factors for extubation failure in patients following a successful SBT.70–73 In general, measures of ventilatory parameters, blood gases, and weaning predictors at the end of the SBT do not discriminate between patients who fail and those who succeed extubation.73–76 Although a recent study suggests that a noninvasive method of measuring work of breathing may predict extubation outcome,77 the decision to take out an endotracheal tube is primarily based on the ability to clear secretions and protect the airway. A weak cough and moderate volume of secretions (eg, requiring suctioning every 1–2 h) are individually and synergistically predictive of extubation failure.70,72 Reduced level of consciousness increases the risk of aspiration, and some suggest a Glasgow coma score of ≤ 8–10 increases the risk for extubation failure.72 The presence of laryngeal edema can be assessed by a cuff-leak test. Although a meta-analysis suggests that the absence of a leak should alert the clinician of a high risk of upper-airway obstruction post-extubation,78 a recent study found no correlation between the test and occurrence of post-extubation acute respiratory difficulties.79 Concern has been expressed that the absence of a leak may lead to unnecessary prolonged intubation in patients who could otherwise be successfully extubated.80
Protocol Development, Implementation, and Ongoing Assessment
Moving evidence-based therapy into routine clinical practice is challenging, and a protocol is a tool that can help facilitate this knowledge transfer. Protocols are often developed and implemented as part of a quality improvement effort.8,81 A multidisciplinary team approach may be used to develop and implement weaning protocols,82–86 and it has been suggested that multidisciplinary collaboration is indeed critical to the success of a protocol.83 While it may be desirable to use a multidisciplinary team to develop a protocol, it may not always be practical. Another approach is to use a multifunctional respiratory care team, comprised of general staff, managers, educators, and the medical director, to develop the protocol. Sharing drafts of the protocol with the ICU medical directors and nursing leadership and soliciting their feedback is still important to craft the final protocol. Doing so may gain similar benefits as having a multidisciplinary team in terms of ensuring all weaning issues are addressed from the unit perspective and giving them ownership of the process. An advantage of this method is that the number of respiratory therapists able to participate on a department quality improvement team is most likely larger than the number who could participate on a more diverse team. This will lead to a large number of enthusiastic champions of the process who can influence their peers and help with “buy-in” of the new practice.87 Another advantage is that all ICUs can be represented in the process. Key decision makers must agree on the criteria used to trigger major steps in the process so that the protocol will be accepted and used in their area. The weaning protocol at the author's institution was developed with the multifunctional respiratory care team model.88,89
Each hospital's quality improvement effort will most likely have a formal process to follow, such as Plan-Do-Check-Act, Lean Thinking, or Six Sigma. Most clinicians do not have formal training in systems thinking and the concepts of process improvement, or in the methods of changing practitioner behavior,90 so it is important that the team leader has this training. Although the actual protocol development process may differ between institutions, tips to successful implementation of weaning protocols have been described in the literature67 (Table 6). Of particular importance is using an evidence-based approach to determine which best-practice elements to include in the protocol and to assure that they are appropriate for the culture of the ICU. Figure 3 is an example of a strategy highlighting the major steps in an evidence-based weaning protocol. Other important steps include gathering baseline data prior to beginning the protocol and monitoring the impact of the protocol on practice. It is recommended that effective behavior-changing strategies, such as using interactive education sessions, engaging opinion leaders in the process, providing reminders, conducting audits, and giving feedback, be used to focus attention on the process and to gain buy-in from clinicians.
Tips for Implementation of Protocols to Maximize the Likelihood of Success in Achieving Both a Change of Behavior on the Part of Healthcare Practitioners and Long-Term Protocol Implementation
Flow diagram of a weaning protocol emphasizing treating the underlying reason for mechanical ventilation, daily monitoring for the earliest indication that the patient is ready to be liberated from the ventilator, a sedation holiday in conjunction with a spontaneous breathing trial, and an assessment of readiness for extubation.
Having a protocol in place does not guarantee that it will be followed. McLean and colleagues reported that, in spite of an evidence-based weaning protocol being in place for over 1 year, compliance was < 2%.85 After engaging in a multidisciplinary performance improvement process that included focus group sessions and educational sessions with physicians, nurses, and respiratory therapists, the revitalized process resulted in compliance improvement (21.2%) and a reduction in unsuccessful extubation (12.7% vs 3.0%). It is important to conduct ongoing compliance monitoring of key steps in the protocol and to provide feedback to clinicians.
As mentioned earlier, identifying the patient's readiness to begin the process of ventilator liberation at the earliest time possible is a key component of the ventilator discontinuation process. Figure 4 shows examples of the many manipulations that may be required to move the patient through the continuum of care. Reducing FIO2 and PEEP to levels that meet weaning readiness criteria should be an ongoing goal for bedside clinicians. Not reducing FIO2 or PEEP in the face of an above target PaO2 may delay the liberation process and expose the patient to unnecessary risk. Ideally, these ventilator manipulations would be part of a ventilator management protocol so that the bedside clinician can make changes in a timely manner.
Various assessments and therapies considered and potentially applied during the continuum of ventilatory support. NIV = noninvasive ventilation. LPVS = lung-protective ventilation strategies. SBT = spontaneous breathing trial. PSV = pressure support ventilation.
Monitoring performance of the protocol involves measures of both process and outcomes. Process measures might include compliance in performing key steps, such as the daily assessment of weaning readiness, as well as the actions taken following a successful passing of the readiness criteria. Adding these key steps to the ventilator flow sheet ensures consistency in charting as well as providing a visual reminder to perform the key step.
Measures of outcome might include duration of mechanical ventilation, rate of unexpected extubation, rate of unsuccessful extubation, and VAP rate. Median is more meaningful than mean duration of ventilation, as it is less influenced by patients with extremely long or short durations. Research studies generally use the number of ventilator-free days during a 28 day period (28-d ventilator-free days), as this measure takes into account those that have a short duration but die, although this measure may not be practical to obtain routinely. Another factor to consider when making comparisons is to adjust for patient severity.91 One method is to compare the actual with an expected duration of ventilation, such as that from a severity indexing system like Acute Physiology and Chronic Health Evaluation, to compute an efficiency ratio.92 It is easier for an inefficient process to show improvement than it is for an efficient process. For example, 2 patients both have an actual duration of ventilation of 4.0 days, but the predicted duration for patient A is 5 days, while it is 3.5 days for patient B. Although the duration was identical for both patients, the process was more efficient with patient A (ratio of 0.80) than it was for patient B (ratio of 1.14).
When measuring the impact of a weaning protocol, intangible elements should also be considered, such as staff perception of the process and personal satisfaction.22
Summary
Current evidence supports using a systematic approach to ventilator liberation, one that includes a daily assessment of weaning readiness and trials of spontaneous breathing in patients meeting the readiness criteria. A weaning protocol driven by non-physician healthcare providers, such as respiratory therapists or nurses, is a powerful standardization tool that has been associated with reduced duration of mechanical ventilation, VAP rates, unplanned extubation, and cost in many institutions. The greatest impact on outcome is gained when a spontaneous awakening trial is done in conjunction with the daily weaning assessment. Checklists, either used as a standalone tool or in conjunction with protocols, help ensure that evidence-based practice care is consistently applied. Regardless of the tools used, monitoring and reporting the compliance of performing key process steps and of appropriate outcomes are necessary to ensure the process is continuously improved.
Footnotes
- Correspondence: Carl F Haas MLS RRT FAARC, Adult Respiratory Care Department, University of Michigan Health System, University Hospital B1-H234, 1500 E Medical Center Drive, Ann Arbor Michigan 48109-0024. E-mail: chaas{at}med.umich.edu.
Mr Haas presented a version of this paper at the New Horizons Symposium, “The Ventilator Liberation Process: A Fresh Look at the Evidence,” at the AARC Congress 2011, held November 5–8, 2011, in Tampa, Florida.
The authors have disclosed no conflicts of interest.
- Copyright © 2012 by Daedalus Enterprises Inc.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.
- 30.↵
- 31.↵
- 32.↵
- 33.
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.
- 60.
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.
- 72.↵
- 73.↵
- 74.
- 75.
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵