Abstract
The bedside chest x-ray (CXR) is an indispensible diagnostic tool for monitoring seriously ill patients in the intensive care unit. The CXR often reveals abnormalities that may not be detected clinically. In addition, bedside CXRs are an irreplaceable tool with which to detect the malposition of tubes and lines and to identify associated complications. Although the image quality is often limited, bedside CXRs still provide valuable diagnostic information. The interpretation of the bedside CXRs is often challenging, and requires extensive radiologic experience to avoid misinterpretation of the wide spectrum of pleural and pulmonary disease. The clinical information is of substantial value for the interpretation of the frequently nonspecific findings.
Introduction
The bedside chest x-ray (CXR) is still today one of the most commonly requested examinations, and remains the cornerstone of diagnosis and monitoring of the intensive care unit (ICU) patient.1–3 Bedside CXRs are essential for detecting malposition of monitor material, for identifying associated complications, and for analyzing the underlying reasons for cardiopulmonary deterioration.1
The limitations of beside CXR are well known and refer to the superposition of soft tissue, pleural and pulmonary disease, as well as tubes and lines. Frequently, the patient is difficult to position, or is unable to cooperate, and the technical equipment is limited.1
After the introduction of digital techniques in the late 1980s, these techniques became readily accepted at the bedside because of the obvious advantages over conventional techniques.4,5 Digital techniques made possible the rapid transmission of images beyond the radiology department, the option for simultaneous review of images by the radiologist and the attending physician, the improved and much more consistent image quality—even under difficult imaging conditions—and, last but not least, the improved availability of prior studies for comparison.
The potential disadvantages of digital techniques include a decline in communication between the attending physician and the radiologist.6 This may result in a decrease in radiology consultations and an increase in inappropriate clinical actions on the basis of misinterpreted images. This underlines the importance of daily multidisciplinary rounds to ensure high-quality patient care.
A systematic approach to the interpretation of the bedside CXR is advisable.1 It should begin with an assessment of the technical quality of the image, because this strongly influences the interpretation. Subsequently, the position of monitor devices, the cardiovascular status, abnormal parenchymal opacities, evidence of barotrauma in ventilated patients, and pleural effusion must be evaluated. For all aspects, comparison with prior studies is absolutely indispensable. The interpretation of the studies is often demanding and should always be done through an interdisciplinary approach with appropriate clinical information. Extensive radiologic experience with ICU patients is necessary to avoid misinterpretations of frequently nonspecific findings.
Indications
The appropriateness criteria for bedside CXRs, published by the American College of Radiology in 2006, stated the following indications:
A daily, routine CXR is indicated in patients with acute cardiopulmonary disorders and in mechanically ventilated patients.
An immediate CXR is indicated after insertion of an endotracheal tube, central venous catheters, pulmonary artery catheters, chest tubes, and nasogastric tubes (http://www.acr.org/ac).
With the current concerns about radiation exposure and increasing financial pressure, the daily, routine CXR is increasingly up for discussion. Multiple studies state that a daily, routine CXR is no longer indicated in the ICU patient.7,8 Some investigators recommend an on-demand strategy to monitor devices and to evaluate whether the clinical condition is deteriorating. The elimination of daily, routine CXR was found to have no negative affect on hospital and ICU mortality, ICU or in-hospital stay, or number of ventilator days.7,8 An on-demand approach would help to reduce work load and radiation exposure to patients and staff, and save healthcare costs. However, it should be noted that important information for the differential diagnostic interpretation of findings in bedside CXRs lies in the comparison with previous CXRs and the short-term development of abnormalities. Too long a time gap between CXRs may cause problems for interpreting the frequently nonspecific findings.
Positioning of Monitor Devices
Central Venous Lines
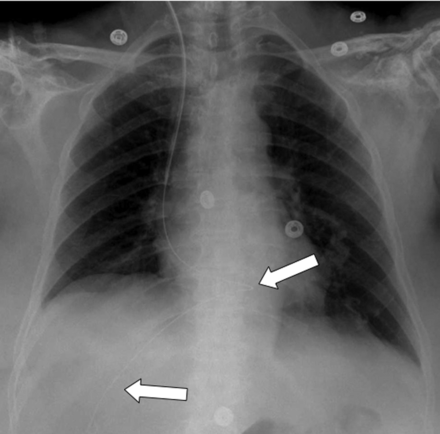
Ideally, the tip of a central venous catheter is located just above the right atrium in the distal superior vena cava (Fig. 1). Malposition is seen in about 10% of controls. The most frequent complication is a pneumothorax in about 6% of patients and is more common with the subclavian than the internal jugular approach.9 A late-appearing pneumothorax should be suspected in patients with respiratory deterioration hours or days after line placement.
Correct position of the central venous catheter: the tip is projected in the distal superior vena cava just above the right atrium.
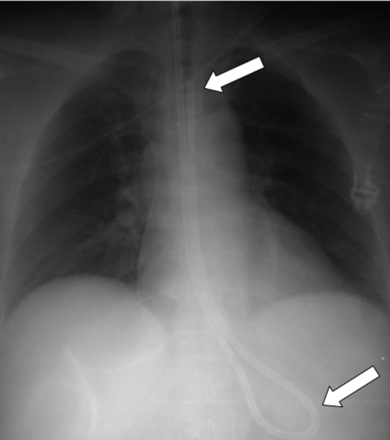
Likewise, after unsuccessful line placement, a CXR is indicated to exclude or show associated complications (eg, mediastinal hematoma, pneumothorax) (Fig. 2). Rapidly progressive mediastinal widening and pleural effusion are signs of extravascular line placement.10 If the catheter is not unambiguously within the expected course of the vein, a malposition should be suspected and confirmed by contrast instillation (Fig. 3). Contrast instillation is recommended in selected cases to exclude extravascular catheter position or malposition in small vessels.
Chest x-ray after unsuccessful line placement. The patient developed a right-sided mediastinal hematoma after unsuccessful insertion of a catheter via the internal jugular vein.
Left: Ambiguous catheter position with rapidly increasing opacity of the right hemithorax. Right: Extravascular malposition is indicated by the extravasation of contrast medium infused into the catheter.
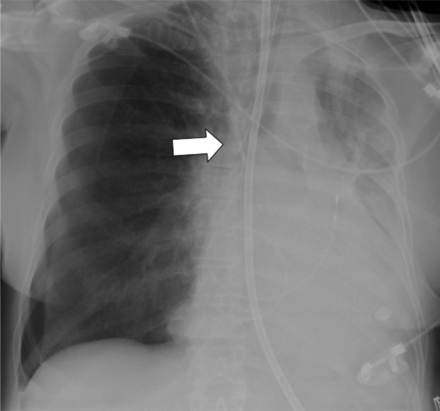
The detection of different malpositions of the catheter requires familiarity with normal and variant thoracic venous anatomy. Positioning of the catheter tip into a small vessel (eg, azygos vein, internal thoracic vein) may cause perforation or occlusion of the vessel (Fig. 4).11 Intraarterial catheter placement should be suspected if the catheter course is atypical (Fig. 5). If the catheter tip impinges perpendicularly against the wall of the superior vena cava, the catheter position should be adjusted, because this catheter position increases the risk of endothelial damage and vascular perforation, hours or even days after insertion (Fig. 6).12 Although the risk is probably low, an intracardiac position of the central venous catheter in the right atrium or the ventricle may lead to valvular or endocardial lesions or cause arrhythmias. Myocardial perforation with hemopericardium and pericardial tamponade is a rare but potentially fatal complication13–15
The tip of the central venous catheter is malpositioned in the azygos vein, indicated by the loop projecting over the azygos vein.
Intraarterial placement of the catheter in the descending aorta is indicated by the atypical course medial to the expected location of the superior vena cava.
Left: The tip of the left subclavian catheter is impinging against the wall of the superior vena cava. Right: Two days later, the catheter perforated the superior vena cava. Subsequently, a so-called “infusion thorax” evolved after 2 L of fluid for parenteral nutrition had run into the chest.
Central venous catheters are typically inserted using the Seldinger technique. The loss of the guide wire is a rare complication of central venous catheter placement, detected by CXR (Fig. 7).16
Chest x-ray may reveal completely unexpected findings, as in this patient with a forgotten wire after line placement in the middle of the night.
Tracheal Tubes
A CXR is indicated immediately after intubation, because malposition is common and may be missed clinically. The appropriate position of the tip of the endotracheal tube is 4–6 cm above the carina with the head in a neutral position.17 The head position is important because movement of the head leads to downward or upward positioning of the tube by approximately 2 cm.18 Malposition of the endotracheal tube occurs in up to 15% of controls.19,20 Most often the tip of the tube is malpositioned in the right main bronchus, which leads to left-sided atelectasis and overinflation of the ventilated lung if the tube malposition remains unrecognized, increasing the risk of barotrauma (Fig. 8). Unilateral intubation is often not detected by auscultation, because up to 60% of patients with mainstem intubation still have symmetric breath-sounds.21
Malposition of the endotracheal tube: the tip has been placed too low in the right main bronchus, with consecutive atelectasis of the left lung and a pneumothorax due to barotrauma on the right side.
A too-high placement of the tip of the endotracheal tube is at risk for spontaneous extubation, aspiration, and injury to the laryngeal structures (Fig. 9).2 Esophageal intubation is a rare, but potentially disastrous complication. It is recognized clinically in most cases by severe hypoxemia and absent respiratory sounds. Radiological signs of esophageal intubation include an endotracheal tube appearing to the left of the contour of the trachea, overdistention of the esophagus and the stomach, and displacement of the trachea by the inflated cuff.21,22
The tube has been unintentionally placed too high, with the tip 9 cm above the carina, and the risk of spontaneous extubation.
Tracheal rupture, usually occurring through the membranaceous part of the trachea, is a rare but serious complication of intubation. Patients may present with a pneumomediastinum, massive soft tissue emphysema, or even a pneumothorax.23 Computed tomography (CT) examination is recommended in suspected tracheal rupture, for precise localization of the rupture site (mostly in the proximal main bronchus) and for planning further treatment (eg, stent insertion, surgery).
Nasogastric Tubes
The optimal position for the nasogastric tube depends on its intended use; if used for feeding, its tip should lie in the gastric antrum. All of the side holes, which are several centimeters distant from the tip of the tube, should be placed within the stomach to prevent aspiration. Malpositions of the nasogastric tube are rare, but potentially fatal if clinically unsuspected.24–27 Following insertion of a nasogastric tube, a CXR is mandatory to confirm correct tube position, at least before infusing nutrient solution. The most common malposition of the nasogastric tube is when it forms a loop within the oropharynx, the esophagus, or the stomach, with upward positioning of the tip (Fig. 10). Resulting complications are a malfunction and aspiration of the nutrient solution. The feeding tube may inadvertently enter the tracheobronchial tree, leading to pneumonia or to bronchial perforation, with subsequent pneumothorax or even a bronchopleural fistula (Fig. 11).28 Gurgling heard over the left upper quadrant after injection of air into the tube may also be detected if the tube is malpositioned in the pleural space or in the esophagus.2,29
Nasogastric tube malposition: the tube doubles back on itself, with the risk of aspiration.
Transpulmonary malposition of the nasogastric tube, with the tip in the pleural space, which resulted in a pneumothorax.
Some nasogastric tubes are only faintly radiopaque, and malposition can therefore easily be missed on the CXR.30 In faintly radiopaque nasogastric tubes, contrast instillation is recommended to control the tip position of the tube.
Chest Tubes
The optimal positioning of a chest tube depends on its indication, whether it is used to evacuate a pneumothorax or a pleural effusion, and on the extent and location of the air or fluid accumulation. The chest tube is inserted anteriorly when it has to drain air, or posteriorly if it has to drain fluid. The side holes of the chest tube should project within the pleural space. A CXR is indicated immediately after insertion to control the position of the tube, to evaluate the efficacy of the drainage, and to rule out associated complications. Malposition of a chest tube includes locations in the interlobar fissure, lung parenchyma, the chest wall, or even the abdomen (Fig. 12).31–33 Malpositioning, especially in the soft tissue of the chest wall, occurs more frequently in obese patients. A malposition should be suspected when the drainage function is inadequate or the pneumothorax or pleural effusion persists.34 CT is, by far, superior to CXR for assessment of drain position and associated complications.31 Possible complications include bleeding due to laceration of an intercostal vessel, the liver, or the spleen (Fig. 13). Malpositioned chest tubes may even injure large mediastinal vessels (aorta, pulmonary vessels, vena cava).
Increasing soft tissue emphysema and persistent pneumothorax indicates malposition of the pleural drain, primarily in the subcutaneous soft tissue of the chest wall in this patient.
This is an example of a rather obese patient in whom a drain was placed blindly for drainage of a right-sided pleural effusion. Left: The drain was not functioning properly and the chest x-ray did not show any decrease in the effusion. Right: Computed tomography revealed an infradiaphragmatic positioning of the drain, with laceration of the liver.
Intraaortic Balloon Pump
An intraaortic balloon pump is placed in patients with severe left ventricular failure to support cardiac pump function. The balloon inflates in diastole and deflates in systole. If the CXR is taken in diastole, the intraaortic balloon pump is detected as an elongated gas-filled structure projecting over the descending aorta. A metallic marker indicates the proximal end of the intraaortic balloon pump. Ideally, the tip of the intraaortic balloon pump is positioned just distal to the origin of the left subclavian artery.11 The optimal position should be confirmed on plain CXRs, with the tip projected between the middle and the lower third of the aortic notch.
A too-proximal location of the intraaortic balloon pump interferes with the origin of the supraaortic arteries and may occlude the arteries supplying the brain, with the consequent risk of cerebral infarction. A too-distal location may interfere with the origin of the visceral arteries and also hampers the function of the balloon (Fig. 14).35–37
A small metallic marker indicates the tip of an intraaortic balloon pump. Left: A too-proximal location of the intraaortic balloon pump interferes with the origin of the left subclavian artery. Right: A too-distal location may interfere with the origin of the visceral arteries and may also hamper the function of the balloon.
Pleural Abnormalities
Pleural Effusion
A pleural effusion is a very frequent finding in ICU patients (more than 60%). It may consist of a transudate, an exudate, blood, bile, or chyle.38,39 In a febrile patient with unilateral (often loculated) pleural effusion, an empyema has to be considered. With the patient in a recumbent position, the detection of a pleural effusion is more challenging than when the patient is in an erect position. In the supine patient position, the pleural fluid accumulates in the dependent postero-basilar recess.40,41
The classic imaging findings of a pleural effusion are a basal hazy opacification without an air bronchogram, an obliteration of the contour of the diaphragm, and blunting of the lateral costophrenic angle (Fig. 15).1 A loculated effusion in the interlobar fissure appears as a homogeneous wedge-shaped or biconvex opacity.
The classic findings of a pleural effusion are a basal hazy opacification without an air bronchogram, an obliteration of the contour of the diaphragm, and a blunting of the lateral costophrenic angle.
Pleural effusions are frequently missed on supine CXRs, especially in obese patients. In addition, the amount of pleural effusion is often underestimated on the bedside CXR; more than 500 mL is necessary to cause a clearly visible opacity on the CXR (Fig. 16).42 A sudden appearance or rapid increase in pleural fluid suggests a hematothorax, especially in trauma patients or after therapeutic or diagnostic thoracic procedures (Fig. 17). The homogeneous opacity of the “grid effect” due to misalignment of the grid should not be confused with a unilateral pleural effusion. The concomitant unilateral increased soft tissue opacity identifies the grid effect as the underlying cause.
The amount of pleural fluid is often underestimated on the chest x-ray, as illustrated here by the computed tomography scan obtained on the same day.
A sudden appearance or rapid increase of pleural fluid suggests a hematothorax, especially in trauma patients or after surgery. The computed tomography scan shows the hyperdense left-sided pleural fluid collection in this trauma patient who suffered multiple dislocated rib fractures after thoracic trauma.
Pneumothorax
A pneumothorax is seen in trauma patients but can also be iatrogenic after line placement or as a result of barotrauma in ventilated patients. The incidence of pneumothorax in ventilated patients varies between 4% and 15%.43,44 In the supine patient position, the classic findings of a pneumothorax are frequently lacking, and the pneumothorax is not diagnosed in up to 30%.43 It is important to diagnose even a small pneumothorax, because, in ventilated patients, it may rapidly develop tension. In contrast to a pleural effusion, air rises to the non-dependent areas of the chest; in the supine position, air is found in anterior and medial locations.44 Radiographic signs of a pneumothorax in the supine patient position are different from those found in the erect position. The radiographic signs include a hyperlucent anterior costophrenic sulcus, a hyperlucency over the upper abdominal quadrant, and an increased visualization of the contour of the diaphragm and the heart (Fig. 18).
In addition to a hyperlucency, mostly seen at the lung base (left), a sharp delineation of the diaphragm or the cardiac contour (right) should raise the suspicion of a pneumothorax.
If the patient develops extensive soft tissue emphysema, it can be impossible to localize or detect a pneumothorax on the CXR (Fig. 19). In the supine patient position the amount of pleural air is often largely underestimated (Fig. 20). Skin folds, a frequent finding, especially in older cachectic patients, should not be confused with a pneumothorax (Fig. 21). Lines that continue over the border of the chest are unsharp, as opposed to the sharp white pleural line or lines that interfere with vascular structures, which do not represent pneumothorax lines. Signs of tension are a mediastinal shift to the contralateral side, the flattening of the cardiac border, the depression of the hemidiaphragm, and the so-called “deep pleural sulcus,” the latter describing a widened and atypically downward-positioned lateral pleurodiaphragmatic sulcus.
If the patient also develops extensive soft tissue emphysema, it can be impossible to localize or detect a pneumothorax on the chest x-ray.
In the supine patient position, air rises to the anterior and medial areas of the chest. Often, typical signs of a pneumothorax are missing (left) and the amount of pleural air is largely underestimated on the chest x-ray, as illustrated by the computed tomography scan (right).
Skin folds, a frequent finding in older cachetic patients especially, should not be confused with a pneumothorax. Lines may continue over the chest wall (left) or vascular structures can run throughout, and a sharp white pleural line is missing (right).
Pulmonary Parenchymal Abnormalities
Atelectasis
Atelectasis is the most common cause of a lung opacity in an ICU patient. The left lower lobe is, by far, most commonly affected in 66% of ICU patients, followed by the right lower lobe (22% of ICU patients), and the right upper lobe (11% of ICU patients).45 In many cases the atelectasis is linear or band-like, located in the dependent areas of the lower lobes. It is important to differentiate between an obstructive atelectasis due to an endobronchial obstruction and a compression atelectasis due to a pleural effusion, a pneumothorax, an enlarged heart, or just the patient's supine position. Segmental, lobar, or complete atelectasis of the entire lung is often caused by obstruction of the corresponding bronchus due to mucus plugging, blood clots, aspirated foreign bodies, or a malpositioned endotracheal tube. Acute endobronchial obstruction from a mucus plug is quite common in ICU patients, and atelectasis can occur very rapidly and change frequently from day to day.
Radiographic signs of atelectasis include an increased opacity, a loss of the contour of the diaphragm and the heart, and an increasing volume loss with a mediastinal shift and displacement of pulmonary fissures. In the recumbent patient position, radiographic signs of volume loss may not be as obvious as in the erect position. Radiographic signs of a plate atelectasis are triangular or band-like parenchymal lung opacities with relatively sharp margins. Atelectasis that persists and becomes larger, with more ill-defined borders, is suspicious for bacterial superinfection (Fig. 22). The bedside CXR is relatively insensitive for the detection of air-space opacities in the basal parts of the lung. The basal opacities identified on CXRs, compared to CT studies, are frequently underestimated.46 The presence or absence of an air bronchogram is helpful in recognizing the cause of atelectasis, and plays an important role in the therapeutic decision-making. If an air bronchogram is absent, the atelectasis is typically caused by an endobronchial obstruction, which might be relieved by bronchoscopy, with consequent respiratory improvement. A positive air bronchogram is usually seen in compression atelectasis, due to a pleural effusion or a pneumothorax. Patients with compression atelectasis will typically not benefit from bronchoscopy, but may require a pleural drainage.
Atelectasis is characterized by band-like parenchymal opacities with relatively sharp borders. Atelectasis that persists and becomes larger, with more ill-defined borders, is suspicious for bacterial infection.
Contrast-enhanced CT might be helpful in differentiating atelectasis from pneumonia. Atelectasis typically demonstrates strong homogeneous enhancement, while pneumonia shows a much weaker, more patchy, inhomogeneous enhancement. An air bronchogram may be present in atelectatic and pneumonic consolidations and is therefore not a helpful sign for differential diagnosis.
Pneumonia
Nosocomial pneumonia is a frequent problem in ICU patients, especially in ventilated patients and in patients with ARDS.47 Nosocomial pneumonia is frequently related to aspiration, and therefore caused by a mix of anaerobic and aerobic Gram-negative organisms. In immunocompromised patients, opportunistic infections have to be considered.48,49
In critically ill patients the diagnosis of pneumonia is often challenging, both clinically and radiographically. Air-space opacities of the lung parenchyma are the hallmark of pneumonia, but they can also be present in atelectasis, aspiration, hemorrhage, or pulmonary edema. Typical radiographic features that favor pneumonia as the diagnosis are patchy areas of consolidation or poorly defined opacities that are often multifocal, without volume loss in the non-dependent areas of the chest. Air bronchograms typically occur in pneumonia, but are not specific (Fig. 23). Radiographic changes in the opacities over days are typical for pneumonic infiltration, in contrast to edema, in which opacities change within hours under therapy.
If an air bronchogram is absent, pneumonia should not be confused with extensive pleural effusion. In this patient, a left-sided opacification was interpreted as a huge pleural effusion and a drain was placed blindly (left). Computed tomography revealed an extensive consolidation caused by pneumococcal pneumonia and only a small pleural effusion (right).
Due to the fear of missing the diagnosis, there is a tendency to over-diagnose pneumonia in the ICU patient, although the majority of lung opacities in the ICU patient do not represent pneumonic infiltration.2 However, the diagnostic accuracy for pneumonia, especially in ARDS patients, is very low.50 Any new or increasing opacity on the CXR of a patient with ARDS is suspicious for a pneumonic infiltration. Particularly in the immunosuppressed patient, a negative CXR does not exclude pneumonia, since CXRs fail to show pneumonic infiltration in up to 40% of cases.
The complications of pneumonia include abscess formation, pleural empyema, and development of a bronchopleural fistula (the latter mostly as a late complication). A pleural effusion that rapidly increases in volume or shows fluid pockets in non-dependent body parts is suspicious for infection. CT mostly provides more information about the volume and the distribution of the pleural effusion, while ultrasound is superior for delineating fibrous septa in the pleural space. Abscess formation is a particular complication of an infection with Gram-negative bacteria (aspiration). An attempt can be made to differentiate bacterial infections from viral or fungal infections. There is, however, no possibility to differentiate various bacterial infections on the basis of the radiographic or CT findings.
ARDS
The radiographic findings in ARDS vary with the stage and the severity of lung injury. In the exudative phase (first 24 h), patchy bilateral air-space consolidations evolve. In the intermediate phase (days 2–7), the patchy air-space consolidations progress to bilateral consolidations, with the development of a “white lung” in severe cases. Air bronchograms are commonly present and the lung volumes are often decreased. In the late or proliferative phase (> 1 week), there is a variable pattern of coarse, reticular, and patchy opacities, with areas of hyperinflation. During an uncomplicated course, the air-space consolidations usually remain stable for several days. Any new opacification or deterioration is more likely to present pneumonia rather than worsening of the ARDS. Pneumonic infiltration is frequently missed because the diffuse air-space consolidation in ARDS obscures the radiographic findings of pneumonia.50
In patients with extensive bilateral consolidations, differentiation between pulmonary edema and ARDS may be challenging based on the radiographic findings alone (Fig. 24). The diagnosis of ARDS is, therefore, typically made in conjunction with clinical parameters (hypoxemia with PaO2/FIO2 < 200 mm Hg).51 Serial CXRs are indicated to identify associated complications (eg, barotrauma) or pneumonia. A barotrauma represents a complication of ventilation with a high end-expiratory pressure.52 Early signs are interstitial emphysema with air inclusions in the central bronchovascular interstitium, and subpleural cystic spaces of overinflation. This interstitial air will be transported to the mediastinum, causing mediastinal emphysema, which thus does not indicate a perforation of the trachea or esophagus. With continuing high pressure ventilation the patient develops a pneumothorax and extensive subcutaneous emphysema.
The differential diagnosis of extensive bilateral consolidations in intensive care unit patients ranges from ARDS and extensive pneumonia to severe alveolar pulmonary edema. In ARDS, a complete homogeneous opacification, described as “white lung,” is typical (left). In pneumonia, more inhomogeneous patchy opacifications are seen (middle). A sparing of the subpleural space is more suggestive for an extensive pulmonary edema (right).
Pulmonary Edema
Pulmonary edema is a frequent cause for admission to the ICU, and it also represents a frequent complication that occurs during an ICU stay. Two types of pulmonary edema can be differentiated, based on the underlying pathophysiology: the hydrostatic edema, due to congestive heart failure, overhydration, or renal failure; and an increased-permeability edema.53
Classic findings in congestive heart failure include widening of the vascular pedicle and vascular congestion with peribronchial cuffing, dilated and unsharp vascular structures, and thickening of the interlobular septa (Kerley B lines) that progresses to a so-called “bat-wing” alveolar edema with bihilar consolidations (Fig. 25). In addition, cardiomegaly and bilateral pleural effusions are typical findings in congestive heart failure. The intravascular volume can be estimated on the bedside CXR by the width of the vascular pedicle.54 A vascular pedicle width of more than 7 cm is used as a sign of increased volume. The width of the vascular pedicle can be influenced by different parameters, including patient position, inspiratory level and ventilator parameters, film-focus distance, and the size of the patient. To avoid misinterpretation of the bedside CXR, it is important to consider these parameters.
A hydrostatic pulmonary edema leads to dilated, unsharp central vascular structures, Kerley B lines, and a so-called “bat-wing” edema with bihilar consolidations. In addition, cardiomegaly and bilateral pleural effusions are typical in congestive heart failure.
On CT, edema can cause a spectrum of findings that imitate pneumonic infiltrations. Typically, there is a combination of interstitial fluid and alveolar fluid, the first causing thickened interlobular septa, the latter causing alveolar filling, which ranges from ground glass to consolidation, depending on the amount of fluid. An alveolar edema shows a preference for the dependent lung parts, although there might sometimes also be a surprising patchy distribution with a mix of normal and dense secondary lobules.
A variety of pulmonary and extrapulmonary diseases may cause increased permeability of the capillary wall, leading to noncardiogenic pulmonary edema (Fig. 26). Radiographically, the distinction between hydrostatic and increased-permeability pulmonary edema can often be challenging.55 A widened vascular pedicle of more than 7 cm, and cardiomegaly with a cardiothoracic ratio greater than 0.55, have proven to be the most accurate criteria for distinguishing hydrostatic from increased-permeability pulmonary edema.54
This is a case of increased-permeability edema due to chemotherapeutic agents administered for colon cancer. The width of the vascular pedicle and the size of the heart are not increased in patients with permeability pulmonary edema.
The clinical presentation, the distribution of the opacities, and the course are important factors for narrowing the differential diagnosis and for distinguishing pulmonary edema from pneumonia or ARDS. In addition, interstitial pneumonia or pulmonary hemorrhage can demonstrate identical radiographic findings that are indistinguishable from interstitial pulmonary edema. In patients with underlying parenchymal changes (eg, emphysema, fibrosis), an atypical distribution of pulmonary edema is often present (Fig. 27). Often, in patients with acute mitral regurgitation, a right upper lobe predominance for pulmonary edema is found and should therefore not be confused with pneumonia.
An atypical asymmetric distribution of pulmonary edema is often present in patients with underlying lung parenchymal changes, as in this patient with emphysema.
Summary
In summary, the bedside CXR is an indispensible diagnostic tool for monitoring seriously ill patients in the ICU. The CXR often reveals abnormalities that may not be detected clinically. In addition, bedside CXRs are an irreplaceable tool with which to detect the malposition of tubes and lines and to identify associated complications. Although the image quality is often limited, bedside CXRs still provide valuable diagnostic information. The interpretation of bedside CXRs is often challenging, and requires extensive radiologic experience to avoid misinterpretation of the wide spectrum of pleural and pulmonary disease. The clinical information is of substantial value for the interpretation of the frequently nonspecific findings.
Footnotes
- Correspondence: Edith Eisenhuber MD, Department of Radiology, Göttlicher Heiland Hospital, Dornbacher Straße 20–28, 1170 Vienna, Austria. E-mail: eisenhuber{at}gmail.com.
The authors have disclosed no conflicts of interest.
- Copyright © 2012 by Daedalus Enterprises Inc.