Abstract
Patients who are chronically critically ill constitute 5–10% of patients with acute respiratory failure but demand a disproportionate share of ICU resources. Epidemiologic studies and clinical trials require definitions for enrollment, and a uniform definition would be ideal to allow comparisons between studies. While a consensus definition exists (≥ 21 consecutive days of mechanical ventilation for ≥ 6 h/d), many study designs have required alternative definitions that include requirement for a tracheostomy, a different period of mechanical ventilation, or admission to a weaning facility. Regardless of definition, studies have indicated that the incidence of chronic critical illness has doubled in recent decades and may double again in the next decade. The overall 1-year survival for chronically critically ill patients is between 40% and 50%, depending on the cohort studied. New clinical prediction rules have been developed to better identify patients who are at high risk and low risk of death. These models could be enhanced by data on functional outcomes for survivors. The healthcare system has been adapting to the increase in chronic critical illness by increasing critical beds in short-term and long-term acute care hospitals, but continued monitoring of resources will be necessary, since the prevalence of chronic critical illness is expected to increase further.
Introduction
The definition of chronic critical illness (CCI) has been approached by investigators and clinicians from a number of perspectives. The clinician recognizes the patient with CCI from a broad clinical view as a patient who has survived acute critical illness or injury but has not yet recovered to the point of liberation from life sustaining therapies. The patient is weak, deconditioned, usually delirious or comatose, and receiving prolonged mechanical ventilation (PMV). Other forms of organ support such as vasopressors, inotropes, and renal replacement therapy may still be required, and the patient may be receiving one of several courses of broad spectrum antibiotics for ongoing or recurrent infections.1–3 A tracheostomy is often in place as well as a feeding tube. These patients endure a prolonged period of discomfort and consume a disproportionate amount of ICU resources. Therefore they have become the subject of important research efforts to guide their management and improve outcomes. Clinical and epidemiological studies and quality improvement initiatives require a clear definition of CCI in order to identify eligible patients.4 An ideal definition for an illness or syndrome should have good sensitivity and specificity for the condition, should be easy to apply using data that is routinely present in the medical record, and should be widely accepted by investigators, clinicians, and administrators. The consensus definition for severe sepsis is an example of a clinical definition that has gained broad acceptance.5 Conversely, studies of ventilator-associated pneumonia use multiple definitions because existing definitions either lack sensitivity and specificity or require invasive procedures to apply.6–8 Having a uniform definition for a condition is not a trivial matter. Clinical trials that use different definitions for a condition are difficult to compare and impossible to combine for meta-analysis. Definitions using complex criteria are impractical to reproduce and make trial results difficult to generalize into clinical practice or apply in large scale quality improvement ventures. A uniform definition for CCI has thus proven to be somewhat elusive. This paper will compare definitions that have been used in studies of CCI and review epidemiological data in the context of how the condition has been defined.
Definitions of Chronic Critical Illness
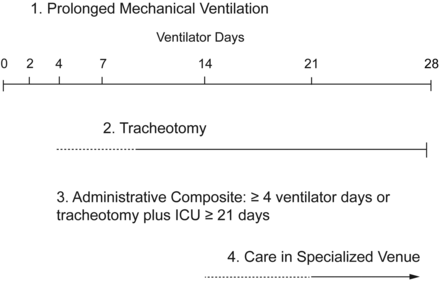
The transition from acute to CCI is gradual, and there is not a clear point of demarcation (Fig. 1).9 The period of transition can vary between patients based upon their premorbid condition and their acute problems. For example, an elderly patient who is already deconditioned due to advanced COPD and who is mechanically ventilated for an exacerbation will give a clinician the impression of CCI after failing spontaneous breathing trials over a period of just 5–7 days. On the other hand, a 24-year-old multiple trauma patient who is recovering from a pulmonary contusion after 12 days and is awake and following commands on the ventilator may not give the impression of CCI. Clinical decision-making by clinicians may differ based upon their impressions of CCI in these 2 cases, such as whether to place a tracheostomy. The elderly patient with COPD may receive a tracheostomy on day 7 of mechanical ventilation because PMV is anticipated, whereas the young trauma patient may not receive one at all since extubation is expected in a few days, and near full functional recovery should follow soon after.
Schematic comparing commonly used definitions for chronic critical illness. Comparisons are based upon the point of demarcation of CCI relative to days of mechanical ventilation.
Defining CCI by Tracheostomy
The clinician-based decision about placing a tracheostomy is one point of demarcation favored by many investigators to identify CCI patients for clinical and epidemiologic trials.10–12 The placement of a tracheostomy can indicate that a physician anticipates that a patient will require PMV and the patient is stable enough to undergo the procedure and likely to survive for a reasonable period of time. The family has consented to the procedure and therefore has indicated that the patient would want ongoing invasive support. The procedure is clearly recognizable in the medical record, and it has its own unique diagnosis-related grouping (DRG) based on procedure codes (DRG 003/004, formerly 541/542 and 483—tracheostomy with mechanical ventilation 96+ hours or principal diagnosis except face, mouth, and neck disease) that facilitates identification of patients in large administrative data sets. For all of these reasons, placement of a tracheostomy for PMV is one of the most common definitions for CCI.
The difficulty with using tracheostomy placement as a point of demarcation for PMV is that the timing of tracheostomy placement is not standard across institutions or even among physicians practicing in the same ICU.13,14 In the example of the young trauma patient given above, the patient would not have met the definition for CCI (received a tracheostomy) if his clinicians elected to follow his course and rate of recovery over a week or more and made decisions accordingly. However, if the patient was in a trauma unit where patients with ARDS and high oxygen requirements routinely receive tracheostomies at day 3 of mechanical ventilation, he would have met the tracheostomy based definition for CCI.
The optimal timing of tracheostomy in critically ill patients has been an issue of intense debate and study in the past decade.15 Despite at least one single center clinical trial showing a mortality benefit for early tracheostomy,16 recent multicenter clinical trials have not demonstrated any mortality benefit.17 Most randomized trials indicate that early tracheostomy is associated with shorter duration of mechanical ventilation and shorter ICU stay. However, as many as 40% of patients in the late tracheostomy groups who met criteria for the study (anticipated PMV) were liberated from the ventilator before meeting the late tracheostomy date,17 so an early tracheostomy approach would result in numerous unnecessary procedures. Some epidemiologic data have indicated that the timing of tracheostomy has shortened over the last decade.11 With the mixed results of clinical trials, practice is likely to remain heterogeneous, therefore affecting the fidelity of tracheostomy as a definition for PMV.
Prolonged Mechanical Ventilation
The other common approach to defining CCI is to use a specific period of mechanical ventilation. PMV is one of the clinical hallmarks of CCI, and it is one of the primary reasons for the high resource utilization associated with the condition. Mechanical ventilation requires ICU care in most acute hospitals, and involves high nurse to patient ratios, therapist time, and equipment costs. Although costs associated with the care of a patient on day 21 of ventilation are less than costs incurred on day 2, costs remain substantial as long as the patient is in an ICU.18,19 Defining CCI according to a period of mechanical ventilation also has advantages of simplicity and uniformity. Days of mechanical ventilation are easy to assess in a medical record, but most administrative databases do not contain days of mechanical ventilation. The major problem with defining CCI as a specific period of PMV is that investigators seem to have very different thresholds for how many days of mechanical ventilation are considered “prolonged.” The term prolonged mechanical ventilation has been used to describe patients receiving ventilation for as few as 2 days to as many as 29 days.20–26 Periods of mechanical ventilation as short as 2 or 4 days include a number of patients who would not even exceed the mean ICU stay for most ICUs. A period of mechanical ventilation as long as 29 days misses a large number of resource intense patients. Participants at a consensus conference in 200427 settled on a definition for PMV as ≥ 21 consecutive days of mechanical ventilation for ≥ 6 h/d. It was felt this definition would have high sensitivity, since most any patient requiring 21 days of mechanical ventilation after acute illness or injury would meet the clinical phenotype recognized by clinicians. This definition was also consistent with the Centers for Medicare and Medicaid Services definitions at the time. To provide some distinction, a group of investigators led by Zilberberg coined the term prolonged acute mechanical ventilation (PAMV) to indicate patients receiving mechanical ventilation for ≥ 96 hours.28,29
Long-Term Mechanical Ventilation
Patients with irreversible neuromuscular diseases who develop ventilatory failure are a unique group. They have not suffered from acute illnesses or injuries that involve systemic inflammation and multi-organ failure. Therefore their outcomes and resource needs are expected to be different than the typical CCI patient, and they are often excluded from clinical and epidemiologic studies of CCI or PMV. They are an important but smaller patient group, and important discussions of their care usually revolve around safe and effective provision of home ventilation rather than recovery from multi-organ failure. The relationships between CCI, PMV, and long-term mechanical ventilation are represented schematically in Figure 2.
Relationship between chronic critical illness (CCI), prolonged mechanical ventilation (PMV), and long-term mechanical ventilation. Most CCI patients require PMV, but not all patients meeting various definitions of PMV would necessarily meet clinical definitions of CCI. Similarly, patients with single organ dysfunction requiring long-term or home ventilation, such as patients with neuromuscular diseases, are unique and relatively few. Most clinical studies of CCI or PMV exclude those patients.
Composite Definitions
To overcome the problem of administrative data sets omitting data on ventilator days, a group of investigators compared 2 approaches for defining CCI (placement of a tracheostomy vs number of days in an ICU) and created a composite approach for use with large data sets. They found that identifying patients by DRG 541/542 (tracheostomy for a condition other than head, neck, or face disease) or by ICD-9-CM (International Classification of Diseases, 9th Revision, Clinical Modification) 96.72 (mechanical ventilation > 96 h) and then limiting patients to ICU stay ≥ 21 days had a sensitivity of 96.7% and specificity of 96.4% for identifying patients who required mechanical ventilation for ≥ 21 days. Another composite approach has recently been advocated by Nelson and colleagues for identifying CCI patients prospectively for clinical trials. They have recommended that patients should be considered to have entered the phase of CCI when they have received at least 10 days of mechanical ventilation and their physician does not expect them to die or be liberated from mechanical ventilation within the next 72 hours.1 This combines a distinct time period with some of the clinical judgment that is employed in decision-making. This definition forms the basis of inclusion criteria for an ongoing randomized controlled trial involving CCI patients.
Mechanically ventilated patients transferred to a long-term acute care (LTAC) hospital or other type of weaning unit are clearly CCI patients, since LTAC admission is reserved for patients requiring prolonged acute care. There is considerable heterogeneity in duration of mechanical ventilation prior to transfer,3 so, depending on the goals of a study of LTAC patients, some standardization of condition or risk may be required.
Epidemiology of Chronic Critical Illness
How big is the CCI population, and why might it be changing? Cohort studies indicate that between 5 and 10% of patients mechanically ventilated in ICUs require PMV.13,30,31 There have been several estimates of the size of the CCI population in the United States. Using the National Inpatient Sample database, Carson and Bach estimated there to be 88,000 patients with CCI in 1997, based upon the prevalence of charges for tracheostomy in patients ventilated for more than 4 days.2 More than half of the patients were over the age of 65, but all age groups were well represented (Table 1). In a statewide population-based study, the incidence rate of tracheostomy for PMV was 24.2 per 100,000 population in 2002.11 This rate had increased by nearly 200% during the previous decade (Fig. 3). This remarkable growth rate was reflected in a more recent analysis of patients with PAMV. Zilberberg and colleagues estimated that, based upon growth trends between 1993 and 2005, the number of PAMV patients is expected to double between 2000 and 2020, from 252,577 to 605,898.28 The number of CCI patients will likely be a third of that number, but the implications are important.
Incidence and In-Hospital Mortality of Chronic Critical Illness, by Age Group in 1997
Incidence rate of tracheostomy for prolonged mechanical ventilation (PMV) per 100,000 population and proportion of mechanically ventilated patients who received a tracheostomy over 10 years in the state of North Carolina (NC). (From Reference 11, with permission.)
There are several lines of speculation as to the reason for the increase in CCI patients. Although elderly patients are at highest risk for CCI, the anticipated growth in population over the age of 65 is only beginning now, so this would not explain recent trends. In fact, recent growth rates have been highest in age groups younger than 65.11,28 One explanation is that patients with chronic illness that predispose to respiratory failure are living longer, and their comorbidities predispose them to PMV. Additionally, advances in the management of acute critical illness have decreased ICU mortality, but patients who would have otherwise died instead recover slowly. Hospital survivors of short-term respiratory failure are often predisposed to recurrence, and they may be more prone to CCI during their next episode. The continuation of these factors, combined with the growth of the population segment over the age of 65, form the basis for the predicted growth of CCI in the future.
There may be reason to hope that some of the factors pushing growth of CCI will attenuate. A number of the acute interventions that improve ICU mortality, such as normal tidal volume ventilation in acute lung injury32 or noninvasive ventilation in COPD exacerbation, also decrease ICU days.33,34 Combining the interventions with daily spontaneous breathing trials and interruption of sedation results in fewer ventilator days and decreases long-term mortality.35 Catheter associated bloodstream infections are a common cause of CCI, and specific measures can reduce the incidence of this complication.36 As these practices become more systematic in ICUs, it is hoped that we will see less severe ARDS, fewer organ failures in severe sepsis, fewer complications of ICU care, and consequently less CCI. Many survivors of short-term critical illness will remain susceptible to recurrence of disease, however. For advances in acute critical care to “pay forward” in terms of less CCI in the long-run, some of these patients must be willing to forego ICU care during their next episode.37,38 That is often not the case in our society, and may not change in the near future.
How prevalent is respiratory failure among the CCI? This question underscores the difficulties of using respiratory failure to define CCI, in which case the answer is 100%. But there are other patients who require prolonged life-sustaining therapies other than invasive mechanical ventilation. An example would be patients with advanced left ventricular failure receiving long-term inotropes or left ventricular assist devices. The number of these patients is growing, but it is still quite small relative to the number of patients who fail to wean from mechanical ventilation. From a resource point of view, an important group of patients are those who have weaned from invasive mechanical ventilation but remain on the verge of ventilatory failure and have difficulty with airway clearance. Occasionally referred to as “medically complex patients,” they still require active respiratory care and high levels of nursing care for prolonged periods. Their numbers are difficult to estimate, but it is notable that more than 2 thirds of patients admitted to LTAC hospitals for prolonged acute care after critical illness are not receiving invasive mechanical ventilation at the time of transfer.39 Respiratory conditions are the most common diagnoses for those patients.
Outcomes
Although by definition CCI patients survive the acute phase of critical illness, hospital and long-term survival remain a challenge. This is due to a preponderance of acute and chronic comorbidities and compromised physical states that make it difficult for them to manage ongoing problems. Reported hospital mortality of CCI is variable, depending on admission and discharge characteristics of the hospitals and definitions used for CCI.12,21,23,25,31,40–45 Long-term survival of CCI patients is more informative for comparisons, because many of the biases that impact studies of hospital outcomes are less relevant.27 Regardless of definition, cohort studies enrolling patients from short-term acute care hospitals indicate that the 1-year survival of CCI patients is between 40% and 50% (Table 2). Outcomes are similar between patients in medical and surgical ICUs, with the exception being trauma patients, who typically have much better survival.42 Comparing studies over time, long-term survival has not improved significantly over the past 20 years.
Outcomes of Chronic Critical Illness by Cohort Definition
From a clinical and administrative point of view, focusing on aggregate outcomes is not always the most productive way to describe survival. Some patients are at much higher risk than others, and distinguishing between high and low risk patients can potentially aid in clinical decision-making and resource management. The ProVent score is a mortality prediction model that uses 4 clinical variables (age, platelet count, requirement for vasopressors, and hemodialysis) measured on day 21 of mechanical ventilation to identify patients who are at high risk and low risk for 1-year mortality46 (Fig. 4). A similar model has been developed for risk stratification on day 14 of mechanical ventilation (unpublished data). The simplicity and reliability of this model may assist clinicians in communicating prognosis to surrogate decision-makers. However decision-makers may also be interested in expected functional outcomes for survivors.
Kaplan-Meier curves by ProVent Score for a multicenter cohort of chronic critical illness patients. The cumulative ProVent score is calculated based upon presence of risk factors on day 21 of mechanical ventilation: age ≥ 65 years – 2 points; age 50–64 years – 1 point; platelets ≤ 150 × 109/L – 1 point; vasopressors – 1 point; hemodialysis – 1 point. (From Reference 46, with permission.)
Survivors of critical illness are often left with physical and cognitive deficits, but patients with CCI tend to be more severely affected41 (Fig. 5). Studies have consistently shown that only 10% of patients with CCI are alive at home and functionally independent after one year.44,47,48 However, from a patient's point of view, it is not clear how physical limitations affect overall quality of life. Some studies of long-term survivors of critical illness indicate that patients who have substantial physical limitations score well on domains of emotional and mental health.20,26,49 This suggests that patients may adapt well to physical limitations after life threatening events if they have good social supports.50 The number of patients with persistent cognitive dysfunction after CCI may be as high as 68%,51 and it is more difficult to assume that this outcome would be satisfactory to patients. With all of these factors in mind, outcome prediction in the future should focus on a composite outcome that includes survival and meaningful function.
Number of limitations in activities of daily living for chronic critical illness patients. The white bars represent patients ventilated for ≥ 96 hours with a tracheostomy. The black bars represent patients ventilated for ≥ 21 days. (From Reference 41, with permission.)
The vulnerable condition of CCI patients has important implications for the healthcare system. Recovery in the index acute hospital and discharge to home occurs in < 10% of cases (see Table 2). A more typical course involves multiple transitions to different levels of institutional care. In one cohort of 126 patients receiving a tracheostomy for PMV, 103 hospital survivors experienced 457 transitions in post-discharge care locations over the course of the year48 (Fig. 6). Despite this degree of health system intervention, death was more likely at the end of the year than survival at home.
Trajectories of care over the first year after discharge for a cohort of patients requiring prolonged mechanical ventilation in a United States tertiary care hospital. Arrows between locations indicate both the direction of patient transitions and the total number of patients transferred between locations over one year. Solid lines represent initial transitions between the hospital and other locations. Dashed lines represent subsequent hospital readmissions and discharges involving post-discharge care locations. Dotted lines represent transitions among post-discharge care locations, including home. Each box summarizes the total numbers of both readmissions and patients admitted, as well as how many patients remained or died in each location of care at one year. * Seven transitions to in-patient hospice and death not shown (3 from the acute hospitalization and 1 each from home, long-term acute care facility, skilled nursing facility, and hospital readmission). One transition from skilled nursing facility to skilled nursing facility not shown. (From Reference 48, with permission.)
Is the health system prepared for either the current or future CCI population? Over the past 2 decades the number of critical care beds has been increasing in United States hospitals, despite an overall decrease in total hospital beds.52 This reflects a shift in hospital resources toward higher acuity patients. During this time there has been a parallel trend of explosive growth of the LTAC industry. The number of LTAC hospitals increased by 8.8% between 1997 and 2006.39 The number of patients admitted to LTACs increased from 63,414 to 103,486. Between 16% and 30% of the patients admitted to these units required mechanical ventilation, and a substantial number of the rest required intensive nursing and respiratory care following an episode of critical illness. The expansion of LTAC hospitals has not been geographically uniform, however, with some areas being under-served and others likely being over-served.53 Furthermore, a moratorium was placed on future expansion of LTAC hospitals in 2008, until the need for their services can be clarified. All of these trends suggest that the healthcare system has been adapting to the growth of CCI patients, but the projected growth in PAMV and consequently CCI indicates that more rapid expansion will be required in the near future (Fig. 7). The healthcare system should have the capacity to adapt further, as long as society is committed to the care and recovery of patients with catastrophic illness. The Centers for Medicare and Medicaid Services is the primary payer for CCI patients. It is imperative that an open dialogue continue between stakeholders in the care of CCI patients and the Centers for Medicare and Medicaid Services. Shortages of critical care beds can have immediate implications for patient outcomes, and adapting to recurrent shortages takes time and forethought.
Projected annual hospitalization days in 10-year increments spent by patients on prolonged acute mechanical ventilation in various strata of hospital care. (From Reference 29, with permission.)
Summary
Investigators and clinicians have not agreed upon a uniform definition for CCI because different study goals and designs often call for unique enrollment criteria. The consensus definition for PMV from 2004 of ≥ 21 consecutive days of mechanical ventilation for ≥ 6 hours per day is a reasonable standard around which other definitions can be based. However, definitions that focus on prolonged mechanical ventilation can include patients who recover quickly and exclude medically complex patients who survive acute critical illness without prolonged mechanical ventilation but still have high respiratory care and nursing needs. Epidemiological studies using various definitions all indicate that the incidence of CCI has been increasing in recent decades. The prevalence of CCI should be monitored in the near future, as aging of the population might be balanced by improvements in the acute management of critical illness and changes in attitudes toward end-of-life care. Epidemiological studies from numerous settings have indicated that 1-year survival from CCI is between 40% and 50% and has not improved significantly over previous decades. More research should focus on quality of life outcomes, which can enhance predictive models and improve discussions of goals of care. The healthcare system has been adapting to increases in CCI, but anticipated growth in the prevalence of CCI will require continued planning to ensure adequate resources.
Discussion
MacIntyre:
You made an interesting point that we may be seeing less so-called fibrotic ARDS as a cause of PMV? Are diagnoses shifting? You mentioned COPD, ARDS, heart failure, trauma. Have the diagnoses that put people on ventilators for 21 days changed over time?
Carson:
That's a good question. You hit the top four. Most cohort studies report ARDS, COPD (often as a comorbidity, if not the primary cause), sepsis, and multi-organ failure in general as being the major risk factors for developing CCI. So are the primary underlying diagnoses shifting? Are there going to be new diagnoses coming to the forefront? I don't think so. Sepsis is just so common; as we get more elderly patients in nursing homes, they're going to be rolling in septic. We're doing a better job of managing it (antibiotics, fluids, etc), but I think it's going to persist along with accompanying acute lung injury as well.
Respiratory therapists are getting pretty darn good—they've always been good, but are getting even better—at managing the long-term ventilator patient with neuromuscular disease. And older populations are going to show up—say, patients with amyotrophic lateral sclerosis—who may be opting for long-term mechanical ventilation, whereas before they wouldn't. Trauma patients and spinal injuries are certainly going to continue. However, I still think the primary diagnoses I noted at the beginning will still be at the top in the foreseeable future.
Hess:
Following up on that, certainly I see more of those patients falling into the neuromuscular disease category, and that kind of messes up your definition a little bit, because many of those patients elect not to have tracheostomies and they are managed long-term on noninvasive ventilation, so they never trip the tracheostomy part of the definition.
Carson:
Yeah, and I think that we might want to start thinking about the two populations a little differently. A patient coming in with single organ failure, without all the other trappings that the ICU doctors typically associate with CCI, is a different patient altogether. The long-term outcome is going to be much better, and their long-term needs are going to be much different, whereas the classic CCI patient's needs are different, the outcome is worse, and challenging in a different way. I think that both patient populations are very important in terms of working out the best ways to care for them, but they're different.
Hess:
Many of those patients may be long-term mechanical ventilation but not long-term critical illness.
Carson:
Exactly.
Nelson:
Most if not all of the recent studies on CCI have excluded patients with neuromuscular disease, so I think the outcomes you're describing are not generalizable to that population. Patients with chronic neuromuscular disease are not an “inflamed” group; that is, they do not have a chronic inflammatory condition.
Carson:
And from a resource point of view—this came out at a Canadian conference last year—it's different. The focus is how to get these patients home safely, as opposed to how to care for the really complex needs of the CCI population outside the ICU. Equally important, but they need to be focused on in different ways.
Muldoon:*
As I'm thinking about how to approach what we'll get out of this conference, both from the question of how are we going to take care of them, and, to a lesser extent, where and who is going to take care of them, I'm struck with the usefulness of the term survivorship of critical illness. It reminds me of 20 years ago, when some of the statistics for stroke recovery or cancer recovery were, in some cases, worse than what we're looking at here, with 50% one-year survival. I'm going to be asking the question where does the entire system—not just intensivists and respiratory therapists—need to go and be built up in the next few years? And what lessons can we learn from stage 4 cardiomyopathy, from stroke that may have recurrence or relapse, and from oncology, which certainly has periods of good function, followed by scattered intermittent decline and re-admissions?
White:
We have also noticed what you alluded to, Shannon, earlier about the reduction in the numbers of patients who appear to be getting tracheotomies. We've done some informal surveys around the Boston area, and physicians working in the ICU setting are much better informed about outcomes from PMV. It may be that those conversations that are happening in the ICUs are now better informed, and therefore families might be making decisions based on a conversation that's more informed. Rather than the conversation that goes, “Yes, we can tracheotomize you; you're going to go to the LTAC and get better.” it's now, “We can tracheotomize you and 50% you might get better and have the tracheostomy tube removed.” So I think that's another variable that's going to impact what's going to happen over the next 5 or 10 years with CCI.
Carson:
That's music to Judy Nelson's ears, I'm sure, as someone who's been trying to focus on improving communication and discussion between patients, families, and caregivers. You might be right, and I'll give you an anecdote. Judy Nelson, Chris Cox, and I are enrolling patients in a clinical trial [ClinicalTrials.gov NCT01230099] about communication at the transition to CCI, and we are looking for patients who have had at least 10 days of mechanical ventilation and who do not expect to be extubated or die within 72 hours. This is a composite definition that we've generated to try and capture patients who are transitioning to CCI, and what we've been finding is that, by the time we start to get on the case at day 10, that conversation has already occurred in a lot of instances. Our intervention was to come in and assist the families through the conversation with a palliative care intervention to support the family and help inform them, and in a notable number of instances the conversation had already occurred. This prompted us to move the intervention date to 7 days for the purpose of our study. The idea is to help the families; we need to catch them before the conversation is held. I think that's different than 15 years ago, when often the conversation didn't occur at all. It was, “They're stuck on the vent: what's the next step? Let's place a tracheostomy and move on.”
White:
We published a paper1 comparing compassionate weaning in the ICU with the LTAC, and we found that the initiation of the conversation on compassionate weaning is much more likely to originate from the family in the LTAC, whereas in the ICU it was more likely to come from the doctors and nursing staff. So I think the families are very much aware and thinking about implications of PMV.
Snyder:†
Your definition was from an administrative data set, and I propose that there's a lot of clinical information out there on some of these patients, and it would be nice to be able to bridge that gap and combine with a clinical data analysis set. Your insightful comment in reference to patients that go into LTAC does need to be looked at: what are their characteristics and traits? Currently and recently the American Hospital Association, the LTAC group has proposed legislation1 to that effect. We look forward to having patient and facility criteria. When you did the scoring at 21 days, did you look at that scoring at any other time?
Carson:
Yes, we took the same variables and measured the variables at day 14 rather than day 21, and then looked at some additional variables as well to see if we could improve the model a bit. So, yes, using those same 4 variables and adding trauma (yes or no) we were able to get a day 14 score. This isn't published yet, but we'll be submitting a paper showing that this score discriminates very well at day 14. We did that in response to our first paper,1 and comments such as, “This is really interesting and helpful, but I want to know sooner.” So we pushed it back to day 14. Can we do the same thing at day 7? I don't know.
Snyder:
As one of the leaders in this type of research, do you see the PMV definition moving from 21 days towards 14 days?
Carson:
It depends on the reason for the study. Again, that's a big point of the talk. It'd be nice to have a single uniform definition for every single study, but, depending on the needs of the study. … I'm an advocate for day 21 as a definition to identify the patient who is CCI, but for the purpose of enrolling patients in our clinical trial, where we're trying to identify patients at risk of transitioning into CCI, we're using a very different definition. For administrative data sets, we're stuck with tracheostomy and 96 hours of ventilation, because that's all that's available in most data sets. It depends on the purpose and goals of your study. What is the outcome you're trying to measure, and in whom? The key is to be very transparent about how you define your population and why.
Mechanick:
It strikes me that this entire meeting hinges on definitions. We will be going back and forth and re-qualifying our conclusions based on this problem. It seems that there is ample precedent in nosology (the classification of disease in medicine) to subtype this medical condition. I would assert that there is a type-1 and a type-2 CCI.
One thing to bear in mind is that CCI type-1 might be a more general form, where the pathophysiology is maladaptation existing at some steady-state set-point. The diagnosis of CCI type-1 would be based on biomarkers, and the therapy would be metabolic.
On the other hand, CCI type-2, as you pointed out, would result from single-organ dysfunction, such as spinal cord injury or neuromuscular disease, and the treatment focus would not be so much metabolic and enlisting the full force of our armamentarium of interventions, but, rather, on that single organ defect to restore the patient back to where they were at baseline.
So, in my mind there's a paradigm of 2 different diseases that are occurring. This confusion contributes to the confusion in the literature and among institutions. I also think we will find cleaner data from our clinical research if the patients are appropriately subtyped.
Carson:
Yes, and I think more data—particularly on the biological side—will help us.
White:
I think we also have to remember that PMV and CCI don't necessarily have to be synonymous.
Footnotes
- Correspondence: Shannon S Carson MD, Division of Pulmonary and Critical Care Medicine, University of North Carolina School of Medicine, 4125 Bioinformatics Building, CB#7020, Chapel Hill NC 27599. E-mail: scarson{at}med.unc.edu.
Dr Carson presented a version of this paper at the 49th Respiratory Care Journal Conference, “The Chronically Critically Ill Patient,” held September 9–10, 2011, in St Petersburg, Florida.
Dr Carson was partly supported by grants from the National Heart, Lung, and Blood Institute and the National Institute of Nursing Research, and has disclosed a relationship with RTI International.
↵* Sean R Muldoon MD MPH, Kindred Healthcare, Hospital Division, Louisville, Kentucky.
↵† Lisa Snyder MD MPH, Select Medical, Mechanicsburg, Pennsylvania.
- Copyright © 2012 by Daedalus Enterprises Inc.
References
- 1.↵