Abstract
BACKGROUND: Flexible bronchoscopic procedures are currently the most often employed technique for demonstrating granulomatous inflammation in sarcoidosis. Conventional transbronchial needle aspiration (TBNA) has been used for over 3 decades; however, it remains an underutilized technique, primarily due to the wide variations in the reported success rates and unconfirmed safety concerns. Herein we perform a systematic review and meta-analysis of studies to estimate the diagnostic yield and safety of TBNA in sarcoidosis.
METHODS: We searched the PubMed and EmBase databases for studies (1980 to January 2012) reporting the efficacy of TBNA in sarcoidosis. The quality of studies was assessed using the QualSyst tool. The efficacy of TBNA in individual studies was calculated as proportions and 95% CIs, and the results were pooled using a random effects model. Heterogeneity and publication bias were assessed for the individual outcomes.
RESULTS: Our search yielded 21 studies (915 patients). The diagnostic yield of TBNA ranged from 6–90%, with the pooled efficacy being 62% (95% CI 52–71%) by the random effects model. TBNA was not associated with any major complication. The diagnostic yield increased to 83% if transbronchial lung biopsy (TBLB) was additionally performed, albeit with increased complications. There was evidence of heterogeneity and publication bias, which significantly decreased on sensitivity analysis after exclusion of retrospective studies.
CONCLUSIONS: TBNA is an efficacious and safe procedure in the diagnosis of sarcoidosis. The performance of TBLB adds to the efficacy of TBNA. Hence, a combination of TBNA and TBLB should be routinely employed in diagnosis of sarcoidosis in those with enlarged mediastinal lymph nodes.
Introduction
Sarcoidosis is a chronic systemic granulomatous disorder of unknown etiology, which most commonly presents with bilateral hilar adenopathy and pulmonary infiltrates. The diagnosis of sarcoidosis requires the presence of compatible clinical and radiological features, along with demonstration of noncaseating epithelioid cell granulomas after exclusion of other known causes of granulomatous inflammation, especially mycobacteria and fungi.1 Bronchoscopic techniques are most often employed for demonstration of granulomas because the lung and hilar/mediastinal lymph nodes are the most afflicted sites in sarcoidosis.2 In fact, flexible bronchoscopy has revolutionized the diagnosis of sarcoidosis, with shift from invasive procedures (like rigid bronchoscopy, mediastinoscopy, and surgical lung biopsy) to a simple day care procedure. The most commonly used bronchoscopy techniques include transbronchial lung biopsy (TBLB), endobronchial biopsy (EBB), and transbronchial needle aspiration (TBNA). Real-time convex probe endobronchial ultrasound-guided TBNA (EBUS-TBNA) has been demonstrated to have an excellent yield in sarcoidosis, due to direct visualization of the lymph nodes beyond the tracheobronchial wall,3 and is currently one of the preferred methods for sampling the lymph nodes in patients with sarcoidosis. However the equipment is expensive and not routinely available.4
TBNA was first developed for use with the rigid bronchoscope;5 however, Wang et al developed a prototype needle for use with the flexible bronchoscope.6 Though widely reported both for malignant and non-malignant conditions, TBNA continues to remain an underutilized technique.7 This underutilization is because of several factors, which include lack of training, variable diagnostic yield, inadequate cytology support, unproven concerns regarding procedural safety, and damage to the bronchoscope.8 This is despite the fact that education and experience have been shown to improve the diagnostic yield of TBNA.9–11
Demonstration of granulomas with exclusion of other causes of granulomatous inflammation is essential in patients with suspected sarcoidosis, especially in countries with high prevalence of tuberculosis. We had previously reported the diagnostic yield of TBLB, EBB, and TBNA in patients with sarcoidosis.11,12 We have also recently reported the efficacy and safety of EBUS-TBNA in patients with sarcoidosis.3 After more than 3 decades of its use, it is high time that the utility of TBNA and its proper place in the diagnostic workup of sarcoidosis is outlined. In this study we performed a systematic review and meta-analysis on the diagnostic efficacy and safety of TBNA in sarcoidosis.
QUICK LOOK
Current knowledge
Flexible bronchoscopy is often employed for demonstrating granulomatous inflammation in sarcoidosis. Conventional transbronchial needle aspiration has been used for over 3 decades, but remains an underutilized technique, primarily due to the wide variations in the reported success rates and safety concerns.
What this paper contributes to our knowledge
Transbronchial needle aspiration is efficacious and safe for diagnosing sarcoidosis, and the efficacy is enhanced by use of a large bore needle and by the addition of transbronchial lung biopsy, although there is a minimal increase in the risk of complications secondary to lung biopsy.
Methods
Search Strategy
We first searched the PubMed and EmBase databases for any systematic review (1980 to January 2012) that had reported the diagnostic efficacy of TBNA in sarcoidosis. No systematic reviews were found. All the authors then independently searched the PubMed and EmBase databases for studies describing the diagnostic value of TBNA in sarcoidosis, using the following search terms: (“transbronchial needle aspiration” AND “sarcoidosis”); (bronchoscopy AND sarcoidosis), and (“transbronchial needle aspiration” OR “tbna”). We reviewed the reference lists of primary studies, reviews, and editorials. In addition, we reviewed our personal files.
We excluded the following types of studies:
Abstracts, editorials, reviews, and case reports
Studies describing diagnostic accuracy of EBUS-TBNA (radial or convex probe) or endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) in sarcoidosis
Studies describing TBNA in ≤ 10 patients with sarcoidosis
Studies in which the denominator (ie, number of patients with final diagnosis of sarcoidosis [granulomas on TBNA or demonstration of granulomas from any site by any methodology and a clinical picture deemed by the clinician to be compatible with sarcoidosis]) was not reported
The criteria for diagnosis of sarcoidosis by TBNA was lymph node aspirates showing epithelioid, noncaseating granulomas or epithelioid and giant cells and absence of identifiable malignancy, lymphoma, or infection (ie, tuberculosis or fungal disease).
Initial Review of Studies
The initial database created from the electronic searches was compiled, and all duplicate citations were eliminated. All authors screened these citations, without blinding, by review of the title and abstract, to capture the relevant studies. Any disagreement was resolved by discussion between the authors. This database was then screened again to include only primary articles, and the full text of each citation was obtained and reviewed. Even where an article was in a language other than English, we retrieved the full text of all these articles, translated them into English (using Google Translate), and extracted the necessary data. Studies were eligible for inclusion if they reported the diagnostic efficacy of TBNA in sarcoidosis.
Data Abstraction
Data were recorded on a standard data extraction form. The following items were extracted:
Publication details: title, authors, other citation details, and geographic location of the study
Type of study: prospective or retrospective
Stage of sarcoidosis
Type of sedation used, diameter and type of TBNA needle, stations sampled, number of lymph nodes aspirated and/or passes made through TBNA, and availability of on-site cytology
Diagnostic yield of TBNA in sarcoidosis, wherein the numerator was the diagnosis of sarcoidosis with TBNA, and the denominator was number of patients with confirmed sarcoidosis
Complications associated with the procedure
Additional yield of combining TBLB with TBNA in the same patient
Assessment of Study Quality
The quality and validity of each article included in this meta-analysis were assessed using the QualSyst tool for qualitative studies.13 This tool consists of 10 questions with scores from 0–2, with the maximum total score being 20. Each study was independently evaluated by 2 authors (RA, ANA) for the stated criteria. The weighted Cohen kappa coefficient was used to determine the inter-observer agreement for selection of studies.
Statistical Analysis
A statistical software package (StatsDirect 2.7.8, StatsDirect, Cheshire, United Kingdom) was used to perform all the statistical analysis.
Determination of the Pooled Effect
We analyzed the diagnostic yield of TBNA by calculating the proportions and 95% CIs for each study, and then pooled the data using DerSimonian weights for the random effects model to derive a pooled efficacy with 95% CI.14
Assessment of Heterogeneity
The impact of statistical heterogeneity on the pooled estimates of individual outcomes was assessed using the I2 test, which measures the extent of inconsistency among the results of the studies. An I2 value ≥ 50% indicates significant heterogeneity.15 Heterogeneity was also assessed using the Cochran Q statistic, and a P value < .1 was considered to be significant.16
Sensitivity/Subgroup Analysis
We planned sensitivity analysis a priori, by using subgroup analysis of prospective versus retrospective studies, due to the limitations associated with retrospective studies and the occurrence of errors associated with the retrieval of information retrospectively from databases.
Assessment of Publication Bias
The presence of publication bias was evaluated using the funnel plot,17 which depicts the proportion on the X-axis against the standard error of the proportion on the Y-axis. In the absence of publication bias, the proportion estimates from studies are expected to scatter near the summary estimate, producing a triangular or funnel shape.
Publication bias was also investigated using 3 statistical tests:
Egger test: detects funnel plot asymmetry18
Harbord test: similar to Egger test, but uses a modified linear regression method19
Begg and Mazumdar test: tests interdependence of variance and effect size using a rank correlation method20
An institutional review board clearance was not required for this study as this was a meta-analysis of published studies.
Results
Our initial database search retrieved a total of 1,355 citations (Fig. 1) of which 21 studies (915 confirmed patients with sarcoidosis) finally met our inclusion criteria.11,21–40 Of these, 7 studies were prospective and 14 retrospective (Table 1). Eleven studies included patients with stage I and stage II sarcoidosis, one study included only patients with stage I sarcoidosis, and the staging was not reported in the remaining studies (see Table 1). The majority of the studies had sampled the paratracheal, subcarinal, and hilar nodes. The larger 19G histology needle was used in 12 studies, and 9 studies used the smaller gauge cytology needles (see Table 1). Two studies employed additional rapid on-site cytology.25,29 The quality of studies was generally good (Table 2), with the median (IQR) QualSyst score being 17 (17–19). The inter-observer agreement for scoring the quality of studies was good (Cohen kappa 0.73).
Citation selection process for the systematic review. TBNA = transbronchial needle aspiration. EBUS = endobronchial ultrasound-guided.
Studies of Transbronchial Needle Aspiration in Sarcoidosis
Qualsyst Tool for Quality Assessment of the Included Studies
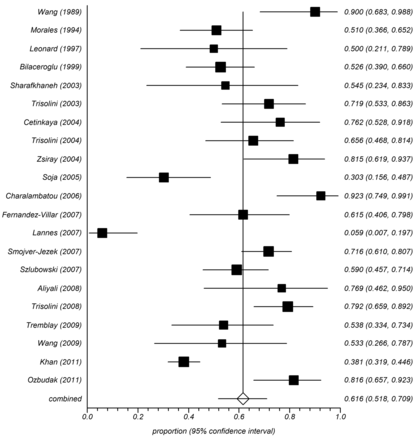
The diagnostic yield of TBNA in sarcoidosis ranged from 6–90%, with the pooled accuracy being 61.6% (95% CI 51.8–70.9%) by the random effects model (Fig. 2). No complication was encountered in the 21 studies, except for minor bleeding at the puncture site. The diagnostic yield was significantly higher (P < .001) in prospective studies (122/171, 71.4%), compared to retrospective studies (393/744, 52.8%). The yield was also higher (P = .002) in studies using a 19G histology needle (236/379, 62.3%), versus those employing smaller gauge cytology needles (279/536, 52.1%). Seven studies (265 patients) reported the yield of TBNA in different stages of sarcoidosis.21,22,24,28,31,32,37 Although the yield was higher in patients with stage I sarcoidosis (106/149, 71.1%), compared to stage II (71/116, 61.2%), it was not statistically significant (P = .09).
Forest plot of the diagnostic yield of transbronchial needle aspiration (TBNA) in sarcoidosis (random effects model). The squares represent the percentage yields and the horizontal lines represent the 95% CIs. The diamond and horizontal line at the bottom represents the pooled prevalence and 95% CI of the pooled prevalence.
There was clinical heterogeneity reflected in the type of study (prospective vs retrospective), inclusion of patients with various stages, variation in the number of passes or lymph nodes sampled, and use of different needles for performing TBNA (see Table 1). There was also significant statistical heterogeneity (I2 88.3%, 95% CI 83.9–91%, Cochran Q statistic 170.74, P < .001). There was evidence of publication bias on visual examination of the funnel plot (Fig. 3). There was also evidence of publication bias on some (Harbord-Egger bias −2.77, P = .06) but not all statistical tests (Begg-Mazumdar Kendall tau −0.124, P = .42, Egger bias 3.42, P = .10).
Funnel plot comparing the proportion versus the standard error of the proportion. The circles represent the individual studies, and vertical line indicates the summary proportion, and the slanting lines represent the 95% CIs. Asymmetry about the pooled line is consistent with the presence of publication bias.
Nine studies (487 patients) also reported the performance of TBLB in the same patients undergoing TBNA.11,22–24,26,28,31,32,37 The diagnostic yield of combined TBNA and TBLB ranged from 58–96% (Fig. 4), with the pooled result being 83% (95% CI 75–89%). The combined yield of TBNA and TBLB (382/487, 78.4%) was significantly higher, compared to TBNA (258/487, 52.9%) alone (P < .001). The yield of TBLB was also significantly higher (P < .001) in patients with stage II disease (75/104, 72.1%), compared to stage I (70/141, 49.6%).22,24,28,31,32,37 Fifteen (3.1%) complications were reported, which included pneumothorax (n = 11, 5 requiring tube thoracostomy), bleeding of 40–100 mL (n = 3), and pneumomediastinum (n = 1). There was no mortality. There was significant statistical heterogeneity for the outcome of combined TBNA and TBLB (I2 72.8, Cochran Q statistic 29.37, P < .001). However, there was no evidence of publication bias on statistical tests (Harbord-Egger bias 2.43, P = .09, Egger bias 1.16, P = .59, Begg-Mazumdar Kendall tau −0.22, P = .36).
Forest plot of the diagnostic yield of combined transbronchial needle aspiration (TBNA) and transbronchial lung biopsy (TBLB) in patients with sarcoidosis (random effects model). The squares represent the percentage yields and the horizontal lines represent the 95% CIs. The diamond and horizontal line at the bottom represents the pooled prevalence and 95% CI of the pooled prevalence.
Sensitivity Analysis
A subgroup analysis was performed, and only prospective studies evaluating the efficacy of TBNA were included (7 studies, 171 patients). The diagnostic yield was significantly higher in the prospective studies (70%, 95% CI 60–80), compared to retrospective studies (P < .001), and there was significant decline in heterogeneity (I2 54%, Cochran Q statistic 13.1, P = .04) and no evidence of publication bias on any statistical test (Egger bias −3.09, P = .13, Begg-Mazumdar Kendall tau −0.52, P = .07, Harbord-Egger bias −1.67, P = .53).
Discussion
The result of this meta-analysis suggests a good diagnostic yield (62%) of TBNA in sarcoidosis, with practically no complications reported in more than 900 patients, indicating that this technique should be routinely employed in diagnosis of sarcoidosis in those with enlarged lymph nodes and where EBUS-TBNA is not available. Moreover, addition of TBLB to TBNA increased the yield to 83%, with a low rate of complications, suggesting that TBLB should also be regularly performed in diagnosis of sarcoidosis. In fact, the diagnostic yield of combined TBNA and TBLB is similar to the recently reported yield of EBUS-TBNA (79%) in sarcoidosis.3 However, the complication rate is higher with combined TBNA and TBLB, compared to EBUS-TBNA alone.
Although histology is not essential in all cases, in the vast majority the diagnosis of sarcoidosis requires the demonstration of non-caseating granuloma and exclusion of other common infections causing granulomatous inflammation.1 A CD4/CD8 ratio > 4 in bronchoalveolar lavage fluid (BALF) has been shown to have a high positive predictive value in diagnosis of sarcoidosis.41 However, not only do the samples require > 15% lymphocytes on differential cell count to be useful,42 but the BALF T cell counts are extremely variable in sarcoidosis, and a ratio > 4 will be seen in < 50% of biopsy proven cases.43 Hence, BALF examination cannot be considered a substitute for histology. Serum angiotensin converting enzyme levels are often elevated in sarcoidosis, but are non-specific, akin to BALF, as common mimics like tuberculosis and other common comorbid conditions like diabetes mellitus show increased serum angiotensin converting enzyme levels.44,45 Gallium-67 nuclear scan findings (panda or lambda sign) can support the diagnosis, but are not seen in all patients and thus cannot replace histology.46,47
Mediastinoscopy is considered the gold standard for sampling mediastinal lymph nodes, with a sensitivity and specificity of 94% and 100%, respectively.48,49 In the past, patients with suspected sarcoidosis underwent mediastinoscopy if TBLB was negative. However, the major caveat is that not all mediastinal nodes can be accessed with this technique, and in one study the sensitivity in sarcoidosis was shown to be about 82%.50 Again, the procedure is not routinely available at all centers, and is associated with major complications, ranging from 1.4–2.3% (including mortality), depending on the experience of the operator.51 In clinical practice, mediastinoscopy is generally considered as the final resort, only if all other techniques fail. Computed tomography-guided transthoracic needle aspiration and/or biopsy can also be used for sampling the mediastinal nodes in sarcoidosis. However, there is a high risk of complications, especially pneumothorax. In a 10-year single center experience of 41 patients with sarcoidosis, the diagnostic yield was 78% with cytology and 96% with core biopsy; however, pneumothorax was encountered in 22% of patients.52
Conventional TBNA is a minimally invasive method of sampling the mediastinal lymph nodes. However, it is an underutilized technique because of many misconceived notions, including poor diagnostic yield and high risk of complications.7,8 The results of this study clearly prove that the technique is fairly efficient and extremely safe. One reason for low yield, especially in sarcoidosis, could be the type of needle (large bore vs small bore) used for performing TBNA, as this study suggests a better yield with the “histology” needle, compared to the “cytology” needle. Hence, the 19G histology needle should be used for performing TBNA in sarcoidosis. Endobronchial ultrasound can improve the diagnostic yield of TBNA by providing “vision” beyond the tracheobronchial wall, and the convex probe allows real time sampling of the mediastinal and hilar lymph nodes. The diagnostic yield (79%) of EBUS-TBNA surpasses any other bronchoscopic investigation (BALF, EBB, TBLB) in diagnosis of sarcoidosis.3 In fact, in the only randomized controlled trial comparing conventional with EBUS-guided TBNA in sarcoidosis, the yield of EBUS-TBNA was significantly higher.38 Thus, wherever available, EBUS-TBNA is preferred over conventional TBNA for sampling mediastinal lymph nodes. However, the technology is fairly expensive, both as an initial investment and per procedure, especially in the developing countries, and is also not available at all centers. Conventional flexible bronchoscopy, on the other hand, is available at most centers, and the results of this meta-analysis suggests that the combination of TBNA and TBLB can provide diagnostic yield approaching EBUS-TBNA. EUS-FNA has shown promise in diagnosis of sarcoidosis.53–55 The limitations of EUS-FNA include inability to perform additional procedures like TBLB or EBB at the same time, and difficulty in accessing paratracheal, hilar, and interlobar nodes, especially on the right side, given the fact that these nodes are usually enlarged in sarcoidosis.
Sarcoid granulomas can involve any part of the respiratory tract, and EBB can also add to the diagnostic yield of TBNA and/or TBLB.12,24,56,57 The yield is highest with abnormal appearing mucosa (54–91%), although 20–40% of normal appearing mucosa can also show evidence of granuloma on histology.12,24,56,57 TBLB is, however, the most commonly performed bronchoscopic procedure in diagnosis of sarcoidosis.58–60 This is because of the fact that even patients with stage I sarcoidosis with normal chest radiographs invariably demonstrate granuloma on surgical lung biopsy.61 The yield of TBLB is higher in stage II versus stage I, as demonstrated in this study and previous studies,58–60 and at least 6 biopsies in stage II and 8–10 in stage I give an optimal yield.62,63 In this systematic review, most of the individual studies performed 4–5 biopsies, which is also the usual practice in our center. Unlike TBNA, TBLB is associated with complications, the major being pneumothorax and hemoptysis. The risk of bleeding following TBLB is higher in immunosuppressed and uremic patients.64 In a study of 540 patients, the complication rate was 0.18% in those who did not undergo TBLB, compared to 2.0% in those who underwent TBLB.65 In another study, the risk of pneumothorax following TBLB was 1.8% when fluoroscopy was used,66 and increased to 2.3–2.9% without fluoroscopy.11,66 In this meta-analysis, the cumulative incidence of major complications due to TBLB in sarcoidosis was about 3%, with none being fatal.
Finally, the results of this meta-analysis are limited by the presence of significant clinical and statistical heterogeneity, although we used the random effects model for minimizing the effects of heterogeneity.15 Another limitation of the analysis was the small number of patients in the individual studies, although we did try to minimize this by including only studies with sample size of at least 11 patients. We also performed sensitivity analyses to investigate the cause of heterogeneity by including only prospective studies, which did decrease the heterogeneity, suggesting that the nature of the study was one of the major factors contributing to heterogeneity.
Conclusions
In conclusion, the results of this study suggest that TBNA is an efficacious and safe procedure in diagnosis of sarcoidosis. The efficacy of TBNA in sarcoidosis is enhanced by use of large bore needle. The diagnostic yield is further enhanced if TBLB is performed in addition to TBNA, albeit at minimal increase in risk of complications. Hence, a combination of TBNA and TBLB should be routinely employed in diagnosis of sarcoidosis, wherever EBUS-TBNA is not routinely available.
Footnotes
- Correspondence: Dheeraj Gupta MD DM, Department of Pulmonary Medicine, Postgraduate Institute of Medical Education and Research, Sector-12, Chandigarh 160012, India. E-mail: dheeraj1910{at}gmail.com.
The authors have disclosed no conflicts of interest.
- Copyright © 2013 by Daedalus Enterprises
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.
- 24.↵
- 25.↵
- 26.↵
- 27.
- 28.↵
- 29.↵
- 30.
- 31.↵
- 32.↵
- 33.
- 34.
- 35.
- 36.
- 37.↵
- 38.↵
- 39.
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵