Abstract
BACKGROUND: High-flow nasal cannula therapy (HFNC) for neonate/infants can deliver up to 10 L/min of heated and humidified gas, and FIO2 can be adjusted to between 0.21 and 1.0. With adults, humidification and actual FIO2 are known to vary according to inspiratory and HFNC gas flow, tidal volume (VT), and ambient temperature. There have been few studies focused on humidification and FIO2 in HFNC settings for neonates/infants, so we performed a bench study to investigate the influence of gas flow, ambient temperature, and respiratory parameters on humidification and actual FIO2 in a neonate/infant simulation.
METHODS: HFNC gas flow was set at 3, 5, and 7 L/min, and FIO2 was set at 0.3, 0.5, and 0.7. Spontaneous breathing was simulated using a 2-bellows-in-a-box model of a neonate lung. Tests were conducted with VT settings of 20, 30, and 40 mL and breathing frequencies of 20 and 30 breaths/min. Inspiratory time was 0.8 s with decelerating flow waveform. The HFNC tube was placed in an incubator, which was either set at 37°C or turned off. Absolute humidity (AH) and actual FIO2 were measured for 1 min using a hygrometer and an oxygen analyzer, and data for the final 3 breaths were extracted.
RESULTS: At all settings, when the incubator was turned on, AH was greater than when it was turned off (P < .001). When the incubator was turned off, as gas flow increased, AH increased (P < .001); however, VT did not affect AH (P = .16). As gas flow increased, actual FIO2 more closely corresponded to set FIO2. When gas flow was 3 L/min, measured FIO2 decreased proportionally more at each FIO2 setting increment (P < .001).
CONCLUSIONS: AH was affected by ambient temperature and HFNC gas flow. Actual FIO2 depended on VT when gas flow was 3 L/min.
Introduction
Via a cannula or nasal prongs, at higher flows than conventional oxygen therapy, high-flow nasal cannula (HFNC) oxygen therapy applied in conjunction with a heated humidifier delivers gas that is almost completely humidified.1,2 In addition to this physiological benefit, HFNC also provides PEEP effects, alveolar recruitment, and increased comfort and tolerance. HFNC is frequently used as a noninvasive mode of respiratory support for neonates and infants with acute respiratory failure.3–6 Higher flow decreases the dilution of oxygen by room air and also washes out anatomical and physiological dead space. This helps to improve fractions of both alveolar carbon dioxide and oxygen. The relationship between HFNC gas flow and the inspiratory flow of the patient, however, influences the actual FIO2. With low-flow oxygen devices, as inspiratory flow increases, actual FIO2 decreases. By contrast, with HFNC, actual FIO2 is stable, at least for adults.7,8 In neonates and infants, however, detailed evaluation of actual FIO2 has been lacking.
During respiratory assistance, adequate heating and humidification maintain mucociliary function, facilitate secretion clearance,9 and help to prevent inspissation of airway secretions, destruction of airway epithelium, and onset of hypothermia. In a bench study of HFNC therapy for adults, we previously reported results for humidification performance: When the inspiratory flow of spontaneous breathing exceeded HFNC gas flow, absolute humidity (AH) was influenced by tidal volume (VT).10 Meanwhile, ambient temperature significantly influenced the amount of condensation in the tubes.11 Because HFNC gas flow in neonates/infants is less than in adults, we conjectured that the AH of gas provided by HFNC to neonates/infants might be lower than expected. In addition, neonates/infants are sometimes put in incubators maintained at 37°C, and it is also usual, for example, after major surgeries to place young patients on an infant warmer. Due to ambient temperature differences inside and outside incubators, when HFNC is applied under these different conditions, the amount of condensation in the HFNC tube may affect the gas temperature and humidity of the delivered gas and decrease these values below expected levels. Because insufficient data have been published to reliably establish relationships between HFNC gas flow, ambient temperature, and VT and HFNC gas temperature and humidity during HFNC for neonates/infants, we designed a bench study to investigate these factors. In addition, we evaluated the relationship of actual FIO2 and VT to gas flow.
QUICK LOOK
Current knowledge
HFNC therapy for neonates and infants can deliver up to about 10 L/min of heated and humidified medical gas to the patient via a wide bore nasal cannula. The beneficial effects of HFNC derive, in part, from better gas warming and humidification than other modes of oxygen delivery: It provides superior humidification and stable FIO2. It has remained unclear, however, whether actual humidity and FIO2 are influenced by ambient temperature or respiratory parameters.
What this paper contributes to our knowledge
The absolute humidity (AH) of supplied HFNC gas was significantly dependent on ambient temperature: Under cooler conditions, AH was lower than expected. AH increased along with increasing HFNC gas flow. As HFNC gas flow increased, the gap between actual FIO2 and set FIO2 narrowed. At gas flow of 3 L/min, at each FIO2 setting increment, as VT increased, measured FIO2 decreased proportionally more. When FIO2 was set at 0.7, with gas flows of 5 and 7 L/min, at VT 40 mL, actual FIO2 decreased.
Methods
Experimental Apparatus
We set up an air/oxygen blender with a flow meter (OA2000, San-You Technology, Ltd, Saitama, Japan), auto-fill humidification chamber (MR290, Fisher & Paykel, Auckland, New Zealand), and heated humidifier (MR850, Fisher & Paykel) (Fig. 1). A breathing circuit for HFNC therapy (RT330, Fisher & Paykel) was connected between the heated humidifier and nasal prongs. We made 2 holes in a polyvinyl chloride cylinder to simulate the external nares of a neonate (width, 5 mm; depth, 4 mm). The prongs of the nasal cannula (OPT314, Fisher & Paykel) were inserted into these holes. The external nares were connected to a 2-bellows-in-a-box model lung (San-You Technology) via a standard ventilator circuit for neonates/infants (DAR 306P8193, Covidien, Mansfield, MA). Mixing of inspired and expired gases was prevented by inserting one-way valves. The whole circuit was placed in an incubator (V-2100G, catalog number 8090508, Atom Medical, Tokyo, Japan). Temperature and relative humidity were measured, in the simulated trachea, with a hygrometer (Moiscope, Senko Medical, Tokyo, Japan). Oxygen concentration was measured with an oxygen analyzer (LZ100, San-You Technology) upstream from the model lung. At the opening of the model lung, flow was measured with a pneumotachometer (4700 series, 0–160 L/min, Hans Rudolph, Shawnee, Kansas) and a differential pressure transducer (TP-602T, ±5 cm H2O, Nihon Kohden, Tokyo, Japan). VT was calculated by digital integration of flow signals. The hygrometer was calibrated at 2 points using a heating and cooling unit (HHC-51, Senko Medical); the oxygen analyzer was calibrated at FIO2 of 0.21 and 1.0, and the pneumotachometer was calibrated using a super syringe. All signals were sampled at 10 Hz/channel using data acquisition software (WinDaq, Dataq Instruments, Akron, Ohio), via an analog/digital converter (DI-148, Dataq Instruments) and stored on a computer.
Experimental apparatus. The high-flow nasal cannula (HFNC) system incorporated an air/O2 blender with flow meter and a heated humidifier. The nasal cannula for HFNC therapy was connected to the manufacturer's standard circuit. To receive the prongs, we made 2 holes in a polyvinyl chloride cylinder to simulate the external nares of a neonate (width, 5 mm; depth, 4 mm). The external nares were connected to a 2-bellows-in-a-box type model lung for neonate via a standard ventilator circuit. In a standard ventilator circuit, one-way valves were connected to prevent mixing of inspired and expired gases. To evaluate simulated VT, a pneumotachometer with a differential pressure transducer was connected between the T-piece and the outlet of the model lung. Humidity of inspired gas downstream of the external nares was measured using a hygrometer. FIO2 was measured downstream of the model lung.
Simulated Spontaneous Breathing
Spontaneous breathing was simulated using a 2-bellows-in-a-box model neonate lung. The model lung comprises 2 bellows in an airtight plastic box. The upper bellows functions as the lung, and the lower bellows functions as the diaphragm. The space between the bellows and box acts as the pleural cavity. The diaphragm bellows is connected to a T-tube, where the Venturi effect of jet flow creates negative pressure inside the diaphragm bellows. This jet flow was regulated to create VT of 20, 30, and 40 mL at 20 and 30 breaths/min. Inspiratory time was 0.8 s with a kind of decelerating flow waveform, and inspiratory peak flow was 2.2, 3.7, and 5.2 L/min.
Experimental Settings
HFNC gas flow was 3, 5, and 7 L/min, and FIO2 was set at 0.3, 0.5, and 0.7. Gas flow was measured with a pneumotachometer with a differential pressure transducer at the outlet of the flow meter. The heated humidifier was set to invasive mode (40°C/−3), and the incubator was set at 37°C. All protocols were also repeated under the same varied conditions but with the incubator switched off.
At each change of experimental setting, we allowed ≥20 min for stabilization. AH of inspired gas and actual FIO2 of expiratory gas were measured for 1 min, and results for the final three breaths were extracted.
Statistics
The results are expressed as mean ± SD. Analysis of variance was performed using repeated measures. All statistical tests were 2-sided, and P < .01 was considered statistically significant. All statistical analysis was performed using commercial software (SPSS 11.01, SPSS, Chicago, Illinois).
Results
The VT of simulated spontaneous breathing was 24.1 ± 2.1, 34.8 ± 2.7, and 43.6 ± 2.2 mL. When the incubator was turned on, the temperature in the incubator was 36.9 ± 0.2°C and AH was 35.5 ± 0.7 mg/L; when the incubator was turned off, the temperature was 26.1 ± 0.6°C and AH was 16.5 ± 1.0 mg/L. When FIO2 was set to 0.3, 0.5, and 0.7, at the outlet of the flow meter, FIO2 was 0.30 ± 0.002, 0.50 ± 0.001, and 0.70 ± 0.003. HFNC gas flow was 3.1 ± 0.1, 5.0 ± 0.1, and 7.1 ± 0.1 L/min.
Effect of Respiratory Parameters on Humidification
AH changed according to the temperature inside the incubator: At all experimental settings, it was significantly higher when the incubator was turned on (P < .001). When the incubator was turned off, AH increased as HFNC gas flow increased (P < .001) but was unaffected by changes in VT (P = .16). When the incubator was turned on, AH was constant regardless of gas flow and VT (Table 1 and Fig. 2); as breathing frequency increased, however, so did AH (P < .001).
Effect of Tidal Volume and High-Flow Nasal Cannula Gas Flow on Absolute Humidity
Effect of respiratory parameters on absolute humidity (AH). At all settings, when the incubator was on, AH was statistically significantly greater than when it was off. When the incubator was off (A), as HFNC gas flow increased, AH increased; when the incubator was on (B), AH was independent of HFNC gas flow and VT. * P < .001.
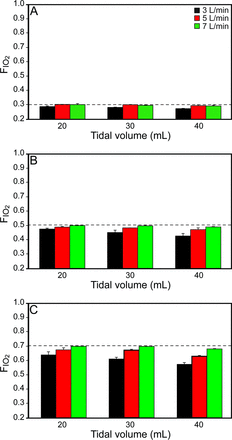
Effect of Respiratory Parameters on Actual FIO2
As HFNC gas flow increased, actual FIO2 more closely corresponded to set FIO2 values; for each increment in FIO2 setting, at 3 L/min gas flow, as VT increased, actual FIO2 decreased (P < .001). When FIO2 was set at 0.7, with gas flows of 5 and 7 L/min, at VT = 40 mL, actual FIO2 decreased (P < .001) (Fig. 3 and Table 2). Breathing frequency did not affect FIO2 (P = .37).
Effect of respiratory parameters on actual FIO2. As HFNC gas flow increased, actual FIO2 reached the values set for FIO2. With flow of 3 L/min, as VT increased, actual FIO2 decreased at each FIO2 setting increment. When FIO2 was set at 0.7, with flows of 5 and 7 L/min, at VT 40 mL, actual FIO2 decreased. Dashed lines represent set FIO2.
Effect of Tidal Volume and High-Flow Nasal Cannula Gas Flow on Actual FIO2
Discussion
In a bench study simulation of HFNC ventilatory assistance for neonates/infants, we investigated the effects of different rates of gas flow, VT, breathing frequency, and ambient temperature on actual FIO2 and AH during HFNC. When the incubator was turned on, internal ambient temperature was maintained at 37°C. We surmise that because vapor inside the inspiratory tube was not lost as condensation, AH was constant regardless of HFNC gas flow and VT. When the inspiratory flow of spontaneous breathing is higher than HFNC gas flow, theoretically, AH would decrease as VT increases because more ambient air is inhaled by the patient. When the incubator was turned on, both the temperature and AH in the surrounding environment were high; consequently, AH was independent of the relationship between HFNC gas flow and VT. When the incubator was turned off, the temperature fell to 26.1 ± 0.6°C, and AH fell to 16.5 ± 1.0 mg/L. Chikata et al12 reported that gas temperature in ventilator circuits that do not have a heater wire fell quickly across a short flow distance, and ambient temperature played a role.11 Moreover, HFNC gas flow for neonates/infants is slower than for adults, and ventilatory gas spends relatively more time inside the circuit. We conjecture that, at the lower ambient temperature when the incubator was switched off, vapor was lost as condensation, and this resulted in lower AH. To prevent the distal temperature probe being warmed by an ambient temperature inside an incubator, it is placed outside an incubator. The distance from the distal temperature probe to nasal prongs is relatively long, and it could be another reason why AH was very low when the incubator was switched off. Inside the incubator, ambient humidity was also lower, and, at high VT, inspired gas could have been diluted by ambient air, which would also reduce AH. Generally, preterm infants and neonates are put inside an incubator during critical illness; however, it is also usual for infants and neonates to be placed under an infant warmer, especially after surgery. Although ambient temperature would not be as high as inside an incubator, it is likely that AH during HFNC oxygen therapy would be lower than expected. In addition, if the distal temperature probe is warmed directly by a ceramic heater, then servo-control would be disrupted, and AH would be greatly reduced. AH could be much lower than would be expected when HFNC is applied for neonates/infants outside an incubator.
By contrast, differences inside the incubator did not influence actual FIO2. Assuming that all of the HFNC gas flow is inhaled, actual FIO2 would depend on the relationship between the inspiratory flow of spontaneous breathing and HFNC gas flow. When inspiratory flow is less than HFNC gas flow, the patient would inhale only the gas delivered via HFNC, and actual FIO2 would correspond with set FIO2. Only when inspiratory flow is greater than HFNC gas flow would the patient inhale both delivered gas and ambient air. When inspiratory time was set at 0.8 s with a decelerating flow waveform and VT was set at 20, 30, and 40 mL, corresponding peak inspiratory flows were 2.2, 3.7, and 5.2 L/min. When HFNC gas flow was 7 L/min, while delivered flow was higher than peak inspiratory flow, measured FIO2 was lower than set FIO2. As VT increased, FIO2 decreased, although not by much. HFNC is an open system, and even with high-flow delivery, it is possible for the patient to inhale ambient air. In line with previous findings, the difference between set FIO2 and measured FIO2 was more apparent when set FIO2 was high because the difference between the set FIO2 and the FIO2 of air (0.21) was greater; consequently, equivalent volumes of ambient air contamination resulted in greater difference.
This study has some limitations. Derived from a bench study, the findings cannot be directly applied to clinical settings. In addition, the present study was done using only one inspiratory flow waveform, and the nasal prongs were firmly fixed into the external nares. In real life, peak inspiratory flow varies both from patient to patient and breath by breath. Finally, the VT of patients with respiratory failure is greater than the VT settings we tested.
Conclusions
In a bench study of simulated HFNC therapy for neonates and infants, we investigated humidification performance and actual FIO2 under different spontaneous breathing and ambient temperature conditions. AH was statistically significantly affected by HFNC gas flow and ambient temperature. When HFNC is used together with infant warming, AH could be lower than expected; we should carefully observe vital signs and phlegm condition when applying HFNC to small infants outside an incubator.
Footnotes
- Correspondence: Masaji Nishimura MD PhD, Critical Care and Emergency Medicine, Tokushima University Graduate School, 3-18-15 Kuramoto, Tokushima 770-8503, Japan. E-mail: nmasaji{at}tokushima-u.ac.jp.
The authors have disclosed no conflicts of interest.
See the Related Editorial on Page 641
- Copyright © 2017 by Daedalus Enterprises