Abstract
BACKGROUND: Positive expiratory pressure (PEP) devices are widely used in clinical settings, yet the performance characteristics of these devices remain relatively unknown. This study compared the performance characteristics of 6 airway clearance devices by varying resistance and flow.
METHODS: Mean PEP, peak PEP, oscillation frequency, and amplitude PEP of the Flutter, Pari PEP S, Acapella Choice, Acapella DM, Acapella DH, and Aerobika devices were obtained across flows of 5, 10, 15, 20, 25 and 30 L/min and at low, medium, and high resistance using an experimental apparatus custom-built for this bench study.
RESULTS: Performance characteristics of the devices differed across flows and resistance settings (device × flow/resistance interaction; P < .001). At a fixed resistance, increasing flows increased mean PEP produced by the Acapella Choice, Acapella DH, Aerobika, and Pari PEP S. Increasing flow resulted in minimal change in mean PEP produced by the Flutter and Acapella DM. Increasing flow increased peak PEP and amplitude PEP produced by all devices except the Acapella DH and Acapella Choice. Increasing flow maintained or increased oscillation frequency for all devices except the Flutter. At a fixed flow, increasing resistance increased mean PEP produced by all devices except the Acapella Choice. Increasing resistance increased peak PEP produced by the Acapella DM, Aerobika, and Pari PEP S but resulted in minimal change in peak PEP for the Flutter and Acapella Choice. Increasing resistance either maintained or increased oscillation frequency for all devices. Amplitude PEP was either maintained or increased during oscillations when increasing resistance for all devices except the Flutter.
CONCLUSIONS: PEP devices produced small but statistically significant variations in performance characteristics across a range of flows and resistance settings. There appear to be flow-dependent and non–flow-dependent devices. Varying flow or resistance typically maintained or increased the production of mean, peak, and amplitude PEP and oscillation frequency.
Introduction
Mucociliary clearance is an essential mechanism of lung defense, enabling the efficient clearance of inhaled particles and pathogens from the respiratory system.1,2 Impaired mucociliary clearance and airway mucus hypersecretion are pathological features of many chronic lung diseases.3 Clinically, chronic cough and excess sputum production are associated with exacerbations4–7 in COPD and cystic fibrosis, hospitalization6 in COPD, declining respiratory function,8 and increased mortality9 in non–cystic fibrosis bronchiectasis. Airway clearance techniques are applied by clinicians to facilitate secretion removal,10–12 optimize respiratory status13 and improve health-related quality of life.14–16 A number of airway clearance techniques are applied in clinical practice including the application of positive expiratory pressure (PEP) devices.17
The addition of positive pressure during prolonged expiration increases expiratory capacity and has been shown to reduce gas trapping.18,19 Studies suggest PEP stabilizes and splints airways open,19–21 and increases intrathoracic pressure distal to retained sections to facilitate movement of secretions centrally for expectoration.21–25 Oscillatory PEP offers the combination of PEP and airway oscillations to alter rheology of secretions12,26,27 and improve secretion transport;12 higher oscillation amplitudes reportedly increase the effectiveness of therapy.28,29 The oscillatory devices aim to approximate cilial movement frequency and pulmonary resonance frequency of 11–15 Hz in adults30–36 to optimize expectoration.33,36
While international guidelines recommend airway clearance techniques,17,37–41 currently there are no definitive studies or guidelines on the preference or superiority of one airway clearance technique compared to another. This ambiguity is the result of heterogeneity in study methodologies and outcome measures used to define the effectiveness of different devices. As a result, the efficacy of airway clearance techniques remains unclear.13,42,43 Despite claims made by manufacturers that their PEP devices perform across a range of flows and resistance settings, the effectiveness of PEP varies in relation to device setting44 and flow; it is therefore important to clarify the differences in the PEP and oscillations generated by different devices when resistance and flow are varied. The purpose of our study was to compare the performance characteristics (ie, mean PEP, peak PEP, amplitude PEP, and oscillation frequency) of 6 commonly used airway clearance devices: Pari PEP S (Pari, Starnberg, Germany), Flutter (Axcan Scandipharm, Birmingham, Alabama), Acapella Choice (DHD Healthcare, Wampsville, New York), Acapella DM (DHD Healthcare), Acapella DH (DHD Healthcare), and Aerobika (Trudell Medical International, Ontario, Canada). The study had 2 aims: first, to compare mean pressure, peak pressure, amplitude of pressure variation, and oscillation frequency of different devices across a range of flows at a fixed resistance setting; and second, to compare the mean pressure, peak pressure, amplitude of pressure variation, and oscillation frequency of different devices across a range of resistance settings at a fixed flow.
QUICK LOOK
Current knowledge
There are a number of positive expiratory pressure (PEP) devices commonly prescribed for airway clearance. The performance characteristics of these devices are not fully understood, and the effectiveness of PEP varies in relation to the device setting and the flow applied to the device.
What this paper contributes to our knowledge
This bench study provides information on the performance characteristics produced by different devices when varying flows and resistance settings. There appeared to be flow-dependent and non–flow-dependent devices. Varying flow or resistance typically maintained or increased the production of mean, peak, and amplitude PEP and oscillation frequency. The clinical importance of the differences between devices is unknown.
Methods
This bench study consisted of a custom-designed experimental setup, and the devices were assessed and compared at different flows and resistance settings. Devices were tested at 5, 10, 15, 20, 25, and 30 L/min, unless otherwise stated. The study was performed at The Prince Charles Hospital in Chermside, Queensland, and at Griffith University, Gold Coast, Queensland, Australia.
Description of Devices and Experimental Conditions
The experimental conditions used to evaluate the devices are detailed in Table 1. Figures of the devices used in this study can be found in Van Fleet et al44 and Berlinski.45
Experimental Conditions Used to Evaluate Each Device Across Different Flows and Resistance Settings
The Pari PEP S consists of a 1-way valve that allows unrestricted inspiration and a resistance to expiration through a fixed (but interchangeable) resistor valve to produce positive airway pressure.46 The resultant positive pressure varies with the resistance setting. The orifice interior diameters of the resistor valve selected were 5, 3, and 1.5 to correspond with low, medium, and high resistance settings, respectively. Because the device is non-oscillatory, mean PEP and peak PEP were the outcomes measured.
The Flutter has a perforated cap that contains an inner conical cavity and steel ball. During expiration, the ball vibrates vertically within its casing, producing PEP and air flow oscillations.47 The angle at which the device is held causes the resistance to expiratory flow to vary. For the purpose of the study, the resistance settings of low, medium, and high correlated to the positions of −10°, 0°, and 20°, respectively.
The Acapella combines the principles of high-frequency oscillations and PEP through the use of a magnetic counterweight.47,48 Three different models of the Acapella were tested. Based on the manufacturer's recommendations, a flow range of 5–15 L/min was tested with the Acapella DM (blue), 20–30 L/min with Acapella DH (light green), and 10–30 L/min with the Acapella Choice. Indicator marks on the dial at the distal end of the devices were used as reference points for resistance settings. The resistance settings of 1, 3, and 5 were tested with the Acapella Choice and correspond with the settings of low, medium, and high resistance. For the Acapella DM and Acapella DH, the middle mark designated medium resistance, and the marks further clockwise and counterclockwise designated high and low resistance, respectively.
The Aerobika is a mechanical device with 5 adjustable resistance settings. During exhalation, a 1-way valve within the device mechanism opens and closes intermittently, resulting in resistance to expiratory air flow and airway oscillations.49 For the purpose of the study, the resistance settings of low, medium, and high were varied by using the settings of 1, 3, and 5, respectively.
Equipment Setup
The devices were tested using the equipment displayed in Figure 1. This custom design was based on the equipment setup used by Volsko et al.47
Experimental setup.
The rig was clamped into place during testing. Silicon connecters linked the device to a pressure transducer (PX138, Omega Engineering, Norwalk, Connecticut). Output from the pressure transducer was connected to a LabVIEW data acquisition system (National Instruments, Austin, Texas), and custom software was used for data collection, storage, and display. The ends of the Y-piece were connected to 2 pressure-compensated medical flow meters (Ezi-Flow, Comweld Group Medical Products, Australia), which allowed accurate flows of 5–30 L/min to be applied to the device. All data were collected at a sample rate of 1,000 Hz. Output from the pressure transducer was filtered using a fourth-order Butterworth, low-pass filter with a cutoff frequency of 40 Hz. For each of the conditions, 9 individual trials were conducted and used for statistical analysis. LabVIEW output from a trial with the Flutter is shown in Figure 2.
Trial figure for the Flutter device at an inclination angle of 0° and 20 L/min flow.
Each trial was at least 5 s in duration with the following output variables extracted: mean trial PEP, peak trial PEP, trial PEP amplitude, and trial oscillation frequency. Data from each of the 9 trials were averaged to determine mean PEP, peak PEP, amplitude, and oscillation frequency.
Performance Characteristics of Devices
Mean PEP is the mean pressure generated when applying air flow. The value depends on air flow resistance imposed by the device, which generates the PEP effect.28 Peak PEP is the highest pressure value generated when applying air flow. Oscillation frequency is the number of oscillations per second when applying air flow. Amplitude PEP is the mean difference of pressure generated between lower and higher values of oscillations when applying air flow.
Statistical Analysis
The performance characteristics of the devices across a range of flows and resistance settings were analyzed and compared using 2-way analysis of variance and post hoc Tukey analysis with commercially available software (SPSS Statistics v25, SPSS, Chicago, Illinois). The probability of a type-1 error was P < .05. Data from each of the trials were normally distributed and expressed as mean ± SD.
Results
Relation Between Performance Characteristics and Varying Flows
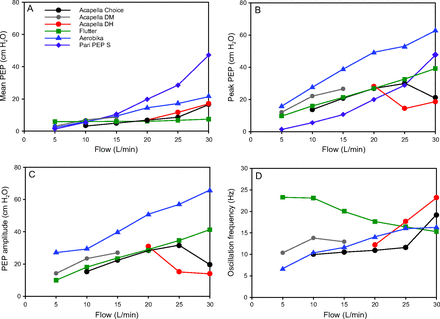
The performance characteristics of different devices across a range of flows and at a fixed medium resistance setting are shown in Table 2 and Figure 3. For each of the performance characteristics, there was a small but statistically significant device–flow interaction (P < .001), which indicates that the performance characteristics of the devices differed across flows.
Mean PEP, Peak PEP, Amplitude PEP, and Oscillation Frequency for Different Devices at Different Flows
Values for mean PEP (A), peak PEP (B), amplitude PEP (C), and oscillation frequency (D) for different devices across flows of 5–30 L/min. All data represent means from 9 trials collected at a fixed medium resistance setting. There was a small but statistically significant device-flow interaction (P < .001) for each performance characteristic. PEP = positive expiratory pressure.
Increasing flows increased mean PEP (Table 2 and Fig. 3A) produced by the Acapella Choice, Acapella DH, Aerobika, and Pari PEP S, with the Pari PEP S demonstrating the greatest increase in mean PEP across flows. Increasing flows resulted in minimal change in mean PEP produced by the Flutter and Acapella DM.
For peak PEP (Table 2 and Fig. 3B), increasing flows resulted in higher peak PEP production by all devices except the Acapella DH and Acapella Choice, for which peak PEP decreased only at higher flows (25 and 30 L/min, respectively). The Aerobika demonstrated the greatest peak PEP across all flows.
Increasing flows maintained or increased oscillation frequency for all devices except the Flutter (Table 2 and Fig. 3D). With the Flutter, oscillation frequency decreased with higher flows, although it still produced the greatest overall oscillation frequency across most flows (5–20 L/min) compared to other devices. The Pari PEP S was not included in this analysis.
Amplitude PEP (Table 2 and Fig. 3C) behaved in a manner similar to peak PEP in that increasing flows resulted in higher amplitude of PEP during oscillations for all devices except the Acapella DH and Acapella Choice, for which amplitude PEP decreased at higher flows (25 and 30 L/min, respectively). The Pari PEP S was not included in this analysis because it is not an oscillatory PEP device.
Relation Between Performance Characteristics and Varying Resistance
The performance characteristics were examined across a range of resistance settings (low, medium, and high) using a fixed flow of 15 L/min; results are shown in Table 3 and Figure 4. Similar to values obtained across different flows at a fixed resistance setting, there was a small but statistically significant device–resistance interaction (P < .001) for each performance characteristic, suggesting that the devices behaved differently across resistance settings.
Mean PEP, Peak PEP, Amplitude PEP, and Oscillation Frequency for Different Devices at Different Resistance Settings
Values for mean PEP (A), peak PEP (B), amplitude PEP (C), and oscillation frequency (D) for different devices across low, medium, and high-resistance settings. All data represent means from 9 trials collected at a fixed flow of 15 L/min. There was a small but statistically significant device-resistance interaction (P <.001) for each performance characteristic. PEP = positive expiratory pressure.
Increasing resistance increased mean PEP produced by all devices except the Acapella Choice, for which there was minimal change in mean PEP when varying resistance (Table 3 and Fig. 4A). The Pari PEP S produced the highest mean PEP across most resistance settings.
Changes in peak PEP across resistance settings are shown in Table 3 and Figure 4B. Increasing resistance increased peak PEP produced by the Acapella DM, Aerobika, and Pari PEP S, but resulted in minimal change in peak PEP for the Flutter and Acapella Choice. The Aerobika produced the greatest peak PEP overall at medium and high resistance, and the Pari PEP S produced the lowest peak PEP overall at low and medium resistance.
Changes in oscillation frequency across resistance settings are shown in Table 3 and Figure 4D. Increasing resistance either maintained or increased oscillation frequency for all devices. The Flutter produced higher oscillation frequencies across all resistance settings compared to the other devices.
Amplitude PEP (Table 3 and Fig. 4C) produced during oscillations increased across resistance settings for all devices but decreased at medium resistance for the Flutter. The amplitude PEP of the Aerobika plateaued at high resistance, although this device produced greater overall amplitude PEP at medium and high resistance settings compared to the other devices.
Discussion
This is the first study to determine and compare the performance characteristics of mean PEP, peak PEP, amplitude PEP, and oscillation frequency for a number of commonly used PEP and oscillatory PEP devices across different flows and resistance settings. These performance characteristics would appear to be essential for the effective use of a device, but it is uncommon to find such information in the manufacturer's marketing or user material.28,29 Only 2 previous studies have compared the physical performance characteristics of several oscillatory PEP devices and found similar performance characteristics between devices.47,50 One study reported on optimal devices and settings required for secretion mobilization,29 whereas another described clinically relevant differences in performance characteristics of oscillatory devices in a simulated model.44 Other studies have only evaluated the performance characteristics of a single oscillatory PEP device.28,35,51–54 Newer airway clearance devices, such as the Aerobika, have a narrow evidence base,44,55,56 and there is general consensus in the literature that further studies are required to evaluate the performance characteristics of airway clearance devices.29,52
For this study, flows were chosen to match those generated by individuals with air flow limitation, and resistance settings were similar to that typically used in clinical practice. For example, if an individual with air flow limitation had a vital capacity (VC) of 2.5 L and expired 50% of VC during a forced expiratory technique, the mean flow would be equal to [(0.5 × 2.5 L)/5 s] × 60 = 15 L/min (ie, the mid-range of flows chosen for the study). Individuals with higher/lower VC who expire a higher/lower percentage of their VC would have flows 5–30 L/min. Notably, the flows chosen for the study are the same as those described by Volsko et al,47 but they differ from those used by Alves et al28 (12–120 L/min), Alves et al52 (12–48 L/min), and Brooks et al53 (48–108 L/min). We chose the flow ranges because lung disease results in a loss of lung elastic recoil and reduction in peak air flow generation. We chose similar angles of inclination for the Flutter based on previous studies.28,44,47,50,51
The evaluation of variability of the mean PEP, peak PEP, amplitude PEP, and oscillation frequency resulted in very low standard deviations. Using our robust experimental setup, we performed repeatable tests and measured flow and pressure waveforms across different flows and resistance settings.
Effect of Varying Flows on Performance Characteristics of Devices
There were small but statistically significant differences in performance characteristics of the devices across flows. The Acapella Choice, Acapella DH, Aerobika, and Pari PEP S appear to be flow-dependent devices because increasing flows resulted in greater mean PEP production; similar findings have been reported in previous studies.28,47,52,54 The Acapella DM and Flutter appear to be non–flow-dependent devices because these devices maintained or produced very minimal changes in mean PEP when flow varied.
The application of pressures higher than atmospheric in the airways during expiration splints airways open20,21,52 and facilitates movement of secretions to larger airways.22–24 Although there are no clear guidelines on the most effective PEP for airway clearance, most studies investigating the effectiveness of PEP therapy document clinically important PEP ranges of 10–20 cm H2O;25,28,53,57 these values have also been shown to improve gas mixing in subjects with cystic fibrosis by reducing alveolar collapse during expiration.21
With the exception of the Acapella DM and Flutter, the devices produced mean PEP of 10–20 cm H2O at mid to higher flows. This differs from results reported by Volsko et al,47 who reported that the Flutter reached these pressures at an inclination angle of 0° only at high flows (25 L/min); Alves et al28 also reported that the Flutter required a flow of 72 L/min for the same inclination angle to produce these pressures. In our study, the Flutter required an inclination angle of 20° and a minimum flow of 15 L/min to reach this mean PEP range of 10–20 cm H2O. The necessity of higher flows may influence the effectiveness of the device when used by individuals with air flow limitation. It is important to note that the Pari PEP S produced the greatest mean PEP across most flows (15–30 L/min) compared to other devices and required the least flow (15 L/min) to reach mean PEP of 10 cm H2O, which may also assist with prescription of the device depending on an individuals' respiratory characteristics. All devices achieved a peak PEP that included the range of 10–20 cm H2O across flows.
The oscillation frequencies of all devices showed frequencies varying from 6–23 Hz, which includes the cited frequency of 11–15 Hz for airway clearance.33,36,58 All devices were oscillatory except the Pari PEP S. All devices maintained or increased their PEP during oscillations (ie, Amplitude PEP) with increasing flows except the Acapella DH and Acapella Choice. It is possible that greater amplitude and oscillation frequencies may increase mucus transport, but the evidence supporting this is lacking.
Higher flows increased or maintained oscillation frequencies for all devices except the Flutter, similar to results reported by Alves et al.52 It should be noted that there was a decline in oscillation frequency when higher flows were applied to the Flutter; however, this device produced frequencies 7–13 Hz higher than other devices across flows of 5–20 L/min. Our results also showed that the Flutter did not reach a mean PEP of 10–20 cm H2O,25,28,53 so total expiratory flow time in patients with lower flows may reduce and use of the Flutter for these patients may not provide clinical benefit.
Interestingly, the Aerobika produced mean PEP and oscillation frequency similar to that provided by other devices, as well as the greatest overall peak PEP and amplitude PEP across all flows. If the aim of therapy were to produce high PEP and amplitude PEP, then this device would appear to produce higher values compared to other devices. The clinical importance of these higher values is unclear.
Effect of Varying Resistance on Performance Characteristics of Devices
There were small but statistically significant differences in performance characteristics of the devices across resistance settings. Consistent with previous studies,47,52,54 increasing resistance maintained or increased the mean PEP produced by all devices. In our study, the Pari PEP S produced the greatest overall mean PEP at medium and high resistance. This may be due to the Pari PEP S being a non-oscillatory device and thus providing positive pressure in a linear fashion, instead of PEP fluctuating with intermittent oscillations.
With the exception of the Acapella Choice, all devices produced a mean PEP > 10 cm H2O at medium to high resistance settings. All devices produced peak PEP > 10 cm H2O across all resistance settings except the Pari PEP S, which required medium to high resistance settings. Interestingly, the Pari PEP S produced the lowest peak PEP at low and medium resistance settings compared to other devices, and the Aerobika produced the greatest peak PEP and amplitude PEP at medium and high resistance settings. Our results are similar to those reported by Van Fleet et al44 for the Aerobika and may assist clinicians when prescribing devices for individuals who require higher resistance settings (ie, individuals with moderate to severe lung disease who require higher PEP to prevent airway collapse and the addition of oscillations to assist with secretion removal). Similar to Alves et al54 and Volsko et al,47 our study found oscillations were maintained or increased when resistance increased.
All of the devices except the Acapella Choice achieved an oscillation frequency of 11–15 Hz across the spectrum of resistance settings. This may be explained with the results reported by Alves et al,52 who found that the device required a minimum flow of 27 L/min at a medium resistance setting to reach these frequencies, whereas the flow used in our study was fixed at 15 L/min. Our results also contrasted Van Fleet et al,44 who reported at low resistance, the Flutter did not produce air flow oscillations, but this is most likely attributed to methodological differences between the studies. Furthermore, pressure was maintained or increased during oscillations (ie, amplitude PEP) as resistance increased for all devices, except the Flutter, which decreased slightly at medium resistance, but this is unlikely to be of clinical importance. Volsko et al47 supports these results for Acapella DM and Acapella DH, whereas Alves et al54 reported that higher resistance settings produced smaller amplitudes for the Acapella DM, which may be due to differences in the instrumentation used.
When increasing resistance, the Pari PEP S performed best for mean PEP, Flutter for oscillation frequency, and Aerobika for peak PEP and amplitude PEP. There are no published studies that have investigated the optimal amplitude PEP in airway clearance devices; however, based on previous studies, we may speculate that higher amplitude PEP may reduce mucus viscoelasticity and enhance the mucociliary transport effect.59 These results provide some evidence for inferences to be made about prescribing these devices for airway clearance.
There are some limitations to this study. Because the aims were to investigate performance characteristics of devices across different flows, the study used a system of constant flow source, and it can be replicated as described in previous studies.28,35,47,51,53 In clinical practice, however, the operational characteristics of the devices change throughout expiration, and so the devices' effect on airway clearance may also differ.
We also chose a single flow of 15 L/min instead of combining flows when comparing devices across different resistance settings. We reasoned that this would be more representative of a patient's steady expiratory flow encouraged in a clinical setting. This does not, however, account for variations in patient effort, airway resistance, or the presence or movement of secretions during expiratory maneuvers.
Conclusion
According to this bench study, there were small but statistically significant differences in the performance characteristics of different oscillatory and non-oscillatory PEP devices. The study allows us to understand the PEP ranges produced by the devices across different flows and resistance settings. There appear to be flow-dependent and non–flow-dependent devices, and varying flow or resistance typically maintains or increases mean, peak, and amplitude PEP and oscillation frequency. The devices produced differences in PEP or oscillation frequency values across different flows and resistance settings, but the clinical importance of this remains unknown. Although based on experimental data, these findings may be useful in future studies verifying the clinical importance of the differences between devices; and in studies that define more precisely the indications and best ways to use the devices when applied to different respiratory conditions.
Footnotes
- Correspondence: Lisa J Franks PT, Physiotherapy Department, The Prince Charles Hospital, Rode Road, Chermside, 4032 Queensland, Australia. E-mail: lisa.franks{at}health.qld.gov.au.
Ms Franks presented a version of this report at The Prince Charles Hospital Foundation research forum, held October 16–20, 2017, in Brisbane, Australia.
The authors have no conflicts to disclose.
- Copyright © 2019 by Daedalus Enterprises