Abstract
BACKGROUND: We tested whether work of breathing in premature newborns estimated by phase angle (θ) by using respiratory inductance plethysmography is decreased during neurally-adjusted ventilatory assist (NAVA) noninvasive ventilation (NIV) versus NIV alone.
METHODS: NAVA NIV and NIV were applied in random order while using respiratory inductance plethysmography to measure the phase angle.
RESULTS: Patient-ventilator asynchrony was decreased during NAVA NIV; however, the phase angle was not different between the modes. A large number of repeated assists with switches to backup were found when using NAVA NIV. Results of the analysis indicated these were due to the apnea alarm limit set during NAVA NIV.
CONCLUSIONS: The improvement in patient-ventilator synchrony supports the hypothesis that work of breathing may be decreased with NAVA NIV; however, we were unable to demonstrate this with our study design. Short apnea time settings with NAVA NIV led to a large number of switches to backup and repeated assists during the same neural effort. (ClinicalTrials.gov registration NCT02788110.)
- Noninvasive ventilation
- neurally adjusted ventilatory assist
- nasal intermittent positive pressure ventilation
- neonatal respiratory distress syndrome
- bronchopulmonary dysplasia
Introduction
Respiratory insufficiency and respiratory failure are frequent sources of morbidity and mortality in premature neonates. Intubation for invasive mechanical ventilation is a life-saving therapy for many of these patients but is not without risks. These risks include pulmonary complications, such as volutrauma, extrapulmonary air-leak syndromes, and traumatic injury to the large airways; non-pulmonary complications, such as retinopathy of prematurity; and long-term complications, for example, bronchopulmonary dysplasia.1,2 Concern over these effects of prolonged mechanical ventilation has led to the development of noninvasive forms of respiratory support.
Noninvasive ventilation (NIV) is a frequently used modality of respiratory support for premature neonates in the setting of respiratory insufficiency or recent extubation. NIV modes that attempt to achieve patient-ventilator synchrony have been shown to be effective at decreasing inspiratory effort compared with unsynchronized NIV and nasal CPAP.3 Synchronizing NIV in premature neonates to the patients’ own respiratory efforts is difficult because of the large air leaks present with any form of NIV as well as the weak inspiratory efforts and high breathing frequencies inherent to this population.4
Neurally-adjusted ventilatory assist (NAVA) uses miniaturized electromyography electrodes attached to a naso-orogastric tube (electrical activity of the diaphragm [EAdi] catheter) to detect diaphragm activation, synchronizing the onset, duration, and peak inspiratory pressure of supporting breaths.5 NAVA can be used with both invasive and NIV interfaces, and several small studies have examined invasive NAVA in neonates.6-12 These studies demonstrated marked improvements in patient-ventilator synchrony with the use of NAVA. 6-12 Fewer studies examined NAVA NIV in this population, and only 2 clinical trials examined NAVA NIV in premature neonates.9-10 Although Lee et al10 reported a lower EAdi swing and maximum values with NAVA NIV, which indicate diaphragmatic unloading and thus possible decreased work of breathing (WOB), other indices of WOB have not been compared in preterm neonates ventilated with NAVA NIV versus other modes of noninvasive support. WOB during assisted ventilation is the portion of the driving pressure for ventilation contributed by the patient’s respiratory muscles. Direct measurement of WOB usually requires the insertion of an esophageal catheter to determine esophageal pressure. The esophageal pressure is a proxy for pleural pressure and is used, along with calibrated tidal volume measurements, to calculate WOB. This approach is invasive and difficult, especially for patients on NIV.13
Research using an animal model has demonstrated that NAVA achieves reduced response time, WOB, and asynchrony with neurally-triggered breaths compared with pneumatically-triggered breaths in intubated pigs with and without lung damage.14 Similar findings were demonstrated in a clinical study of pediatric subjects with bronchiolitis who were intubated.15 Another study examined WOB in neonatal pigs by using a leak-free mask and by comparing NAVA NIV with NIV before and after surfactant depletion. Pressure-time product, an indicator of WOB, was lower with NAVA NIV in both the undamaged and surfactant depleted lungs.16 Pressure-time product cannot be reliably used in infants on NIV because the necessary measurements are not possible with nasal interfaces, which allow large and inconsistent air leaks at the nose and mouth. Another animal study demonstrated that animal-ventilator synchrony was preserved with NAVA NIV and that, by increasing the NAVA level by using a noninvasive interface, WOB (measured by EAdi and esophageal pressure changes) could be decreased to levels seen during invasive ventilation before lung injury.17
Thoracoabdominal asynchrony, which can be measured without invasive monitoring, is an important correlate of WOB and increased respiratory load in preterm infants. Thoracoabdominal asynchrony is measured by using respiratory inductance plethysmography bands around the patient’s chest and abdomen to quantify chest-wall and abdominal movement. The degree of asynchrony between the 2 compartments is reflected in the phase angle (θ), which can be calculated from the respiratory inductance plethysmography band measurements.18 A higher phase angle reflects greater thoracoabdominal asynchrony and increased WOB.
The primary objective of this study was to examine the effect of NAVA NIV versus NIV on estimated WOB in premature neonates with respiratory insufficiency who were receiving noninvasive respiratory support. The effect was examined by comparing the phase angle when using respiratory inductance plethysmography as an estimate of WOB. The secondary objectives of the study were to evaluate the effect of NAVA NIV versus NIV on several respiratory parameters, including breathing frequency, transcutaneous oxygen and carbon dioxide, oxygen saturation and 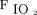 requirement, and EAdi.
requirement, and EAdi.
QUICK LOOK
Current knowledge
Noninvasive ventilation is an effective way of supporting neonates with respiratory distress and an alternative to invasive ventilation. Neurally-adjusted ventilatory assist improves patient-ventilator synchrony when used with invasive or noninvasive ventilation. Improving patient-ventilator synchrony produces favorable changes in ventilatory parameters for neonates receiving invasive or noninvasive support.
What this paper contributes to our knowledge
Improving patient-ventilator synchrony did not decrease work of breathing (measured by using respiratory inductance plethysmography) in neonates supported by using neurally-adjusted ventilatory assist administered via a high-flow nasal cannula. The apnea time setting measured the time period from the beginning of the breath, which creates more asynchronous backup inflations in neonates when using short apnea times.
Methods
As previously described, infants were recruited at a level IV neonatal ICU from 2016 to 2017, the protocol was approved by the institutional review board, and informed consent was obtained from the parent(s) before study procedures.19 The protocol was made available on clinicaltrials.gov (NCT02788110).
Subjects
Infants were eligible for inclusion if they had a current weight between 1 and 2 kg, gestational age at birth between 24 and 34 weeks, respiratory insufficiency that currently required NIV, current 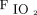 requirement < 0.40, and were clinically stable. Infants with known major congenital anomalies (abdominal wall defects, gastrointestinal tract defects, cleft palate, or neurologic defects), nitric oxide use, or cyanotic congenital heart disease were excluded.19
requirement < 0.40, and were clinically stable. Infants with known major congenital anomalies (abdominal wall defects, gastrointestinal tract defects, cleft palate, or neurologic defects), nitric oxide use, or cyanotic congenital heart disease were excluded.19
Study Protocol
Infants were monitored by using respiratory inductance plethysmography bands (Respitrace Plus, Noninvasive Monitoring Systems, Miami Beach, Florida) placed around the infant’s chest and abdomen, and an oro-nasogastric catheter equipped with electrodes to detect the electrical activity of the diaphragm (EAdi catheter, Maquet Critical Care, Solna, Sweden), while they were ventilated with SERVO-i ventilators equipped with NAVA NIV software version 7.00.04 (Maquet Critical Care) by using a high-flow nasal cannula (RAM, Neotech, Valencia, California) as the nasal interface.19 A calibrated transcutaneous monitor (TCOM4, Radiometer, Brea, California) was attached to the abdomen or chest, and an oxygen saturation probe to detect both heart rate and oxygen saturation was placed on an extremity. Once stable after instrumentation, the infants received 15-min trials of NAVA NIV and NIV in random order, with the first 10 min after device change considered a stabilization period and the last 5 min used for data analysis. Unsynchronized NIV was administered by using the NIV-pressure control mode on the ventilator.
The ventilator settings used during the study periods are summarized in Table 1. Subjects’ current settings (as prescribed by the subjects’ attending physician) were used for the NIV and backup NAVA NIV settings. The same PEEPs were used during both NAVA NIV and NIV. Minimum EAdi values were not used to titrate PEEP. Ventilator leak compensation was used in both modes of support. The NAVA level was selected to achieve appropriate EAdi peak values (10-20 μV). For subjects who were currently ventilated on NIV, a starting NAVA level of 1.5 was used. All the subjects had appropriate EAdi peak values on this NAVA level, which was used during data acquisition. For subjects who were on NAVA NIV before the study, the current NAVA level was used (range, 1.2-2.6), with one exception.
Noninvasive Ventilation Settings
One subject currently ventilated on NAVA NIV had peak EAdi signals that were depressed on the current NAVA level. For this subject, a NAVA level of 1.5 achieved EAdi peaks of 10–20 μV, and this level was used during data acquisition. The apnea alarm limit, or apnea time (delay until backup breath initiation), was set at 2 s (8 subjects) or 3 s (7 subjects), the EAdi trigger was 0.5 μV, and the set maximum peak pressure was 30–40 cm H2O.
Data Acquisition and Measurements
The MP100 Biopac data acquisition system (Biopac Systems, Goleta, California) was used to acquire data from monitoring devices, whereas the RS232 interface and Servo Tracker V.4.0 software (Maquet Critical Care) were used to acquire data from the ventilator. We evaluated measures of patient-ventilator asynchrony by using the NeuroSync index, a standardized automated measure of patient-ventilator interactions.20 The following data were acquired: heart rate, neural breathing frequency, oxygen saturation, transcutaneous CO2 and O2, PEEP, ventilator flow and pressure, rib cage and abdominal respiratory inductance plethysmography signals, and EAdi values (maximum, minimum, and swing).
The data recorded from the rib cage and abdominal respiratory inductance plethysmography bands were used to calculate the phase angle as an estimate of WOB. By producing voltage changes proportional to the change in the cross-sectional area, respiratory inductance plethysmography bands reflect volume changes in the underlying thoracic and abdominal compartments. Calibration was not required because the outcome of interest, the phase angle, involves the relative motion and timing of the 2 compartments rather than the absolute volumes. Thoracoabdominal asynchrony expressed by the phase angle reflects the delay in outward motion of the rib cage compared with the abdomen and is expressed in degrees when assuming the total respiratory cycle is 360°. A phase angle of 180° indicates complete asynchrony, whereas a phase angle of 0° indicates complete synchrony of the abdomen and chest-wall movements (Figs. 1 and 2).
Ten seconds of noninvasive ventilation (NIV). Phase angle calculation NIV data screenshot: note top graph rib cage (red waveform), and abdominal movements (blue waveform) are out of phase. Compare with neurally-adjusted ventilatory assist (NAVA) NIV in Fig. 2.
Ten seconds of neurally adjusted ventilatory assist (NAVA) noninvasive ventilation (NIV). Phase angle calculation NAVA NIV data screenshot: note top graph rib cage, red waveform; and abdominal movements, blue waveform; are only slightly out of phase. Compare with NIV in Fig. 1. There are 2 waveforms for electrical activity of the diaphragm (EAdi), because these data were collected from 2 sources, the ventilator and via Biopac, and the waveforms were used to synchronize the data. The differences are due to the sampling rate of the Biopac system.
Respiratory inductance plethysmography data were downloaded into Microsoft Excel (Microsoft, Redmond, Washington) for analysis. One of us (MDW) composed a program in Excel to analyze each breath and measure the time shift between the abdomen and chest-wall movements as detected by the respiratory inductance plethysmography bands, then divide the time shift by the duration of the respiratory cycle and multiply this by 360 to obtain the phase angle. The equation for the phase angle (θ) is as follows:
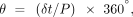 where δt represents the time shift between the 2 sine waves, and P is the wave period or cycle time. Each breath occurring in the 5-min data collection period was analyzed.
where δt represents the time shift between the 2 sine waves, and P is the wave period or cycle time. Each breath occurring in the 5-min data collection period was analyzed.
One of us (NC) designed a program in Excel to analyze data recorded from the ventilator by using the Servo Tracker. This program analyzed the data for patient-ventilator synchrony, generating the NeuroSync index as previously described by Sinderby et al.20 The NeuroSync index uses the EAdi signal and pressure and volume measurements from the ventilator to describe patient-ventilator interactions, and defines this as a percentage.20 This index is calculated by averaging the absolute values of the errors that exist between ventilator and neural breathing cycles, which thereby accounts for trigger error, cycle-off error, and asynchronous events, such as auto-triggering, EAdi without assist (wasted efforts), and multiple EAdi with a single assist.20 A lower percentage indicates fewer total asynchrony events or improved patient-ventilator synchrony.
Statistical Analysis
Data collected were checked for outliers and extreme values as well as distributional assumptions of the parametric statistical tests. A 2-sample paired t test was used to compare the primary and secondary outcomes under the 2 ventilation methods when such assumptions were met. When significant deviation from assumptions was encountered, or when the outcomes are expressed as percentages, one-sample Wilcoxon signed-rank test was used to test the difference from zero between the 2 modes. Statistical analyses for this article were generated by using SAS/STAT software V.9.4 (SAS Institute, Carey, North Carolina) or Stata software V.15 (StataCorp, College Station, Texas). Given the relatively small sample size, the degrees of freedom were insufficient to estimate treatment, carryover, or the period effect. These are aliased with each other. As demonstrated in an animal model and preterm neonates, the treatment effects of NAVA NIV and NIV are relatively short;10,16 therefore, the carryover effect is reasonably assumed to be negligible by including a washout period of 10 min of stabilization. The randomization of the order of the 2 treatments is sufficient to eliminate the period effect; therefore, the analysis focused on estimating the treatment effect in a paired match design, and using a paired t test was appropriate.
Sample Size and Power Calculation
Based on a previous study conducted by Jones et al,16 in a pig model, a 30% reduction in the primary outcome was expected. Because the average phase angle was previously found to be variable among preterm infants (ranged from 5.8°-162.9°), we used several sample size and power calculations.21 A sample size of 15 infants achieves 82% power to detect a 30% change in the primary outcome, with an estimated SD of ±18.8, ±37.5, or ±56.3 for an average phase angle of 50°, 100°, and 150°, respectively, with the use of the NIV mode. All calculations assumed a significance level of 0.05 when using a 2-sided paired t test.
Results
The 15 infants enrolled in this study were receiving NIV, with a mean ± SD  requirement of 0.32 ± 0.06 (range, 0.21–0.39). All the infants were receiving caffeine. The mean ± SD gestational age at birth was 27 ± 2 weeks, mean ± SD chronologic age at study was 40 ± 18 d, mean ± SD postmenstrual age at study was 32 ± 2 weeks, mean ± SD birthweight was 908 ± 223 g, and the mean ± SD study weight was 1,472 ± 372 g. Additional details of the study population were previously described.19
requirement of 0.32 ± 0.06 (range, 0.21–0.39). All the infants were receiving caffeine. The mean ± SD gestational age at birth was 27 ± 2 weeks, mean ± SD chronologic age at study was 40 ± 18 d, mean ± SD postmenstrual age at study was 32 ± 2 weeks, mean ± SD birthweight was 908 ± 223 g, and the mean ± SD study weight was 1,472 ± 372 g. Additional details of the study population were previously described.19
Results for primary and secondary outcome measures are summarized in Table 2. There was no difference in the phase angle with NAVA NIV versus NIV. Maximum and swing EAdi were not different between the modes. The NeuroSync index was lower during NAVA NIV (21 ± 10%, mean ± SD) than during NIV (78 ± 7%), which indicated significantly improved patient-ventilator synchrony (P < .001). EAdi without assist accounted for most of the asynchrony events in the NIV mode. Repeated assists during the same neural effort accounted for most of the asynchrony in the NAVA NIV mode. With an apnea time of 2-3 s set on the ventilator, repeated assists in back-up mode occurred during neural efforts and accounted for a greater percentage of ventilator breaths during NAVA NIV (mean ± SD, 2.6 ± 2.2%) than NIV (mean ± SD, 0.3 ± 0.6%) (P = .001). This study was not powered to detect a difference in performance between the 2 apnea times used, but there was a trend toward more repeated assists during the study period when using an apnea time of 2 versus 3 s (mean, 17.3 vs 9.3; median, 10 vs 10; range, 67 vs 7 for 2 vs 3 s). Other outcomes were similar between modes, with the exclusion of  , which was 0.01 higher during NIV (P = .03).
, which was 0.01 higher during NIV (P = .03).
Respiratory Outcomes
Discussion
Our study confirmed findings of previous studies that showed that NAVA improves patient-ventilator synchrony and that this synchrony is preserved when using noninvasive interfaces.8-10 We were unable to show an improvement in thoracoabdominal asynchrony or WOB when using calculated phase angles. We also were not able to show clinically relevant changes in other outcomes when using transcutaneous monitoring and pulse oximetry. The 0.01 decrease in  during NAVA NIV does not represent a clinically relevant change. There was a trend toward decreased peak inspiratory pressure with the use of NAVA, which did not reach statistical significance.
during NAVA NIV does not represent a clinically relevant change. There was a trend toward decreased peak inspiratory pressure with the use of NAVA, which did not reach statistical significance.
Our findings conflict with studies that used NAVA in similar populations, which demonstrated decreases in  , peak inspiratory pressure, transcutaneous CO2, and EAdi peak.6,12,22,23 These studies, however, were of subjects who were intubated. Lee et al10 performed a study in a similar population by using a noninvasive interface and showed improved patient-ventilator synchrony and lower EAdi peak and EAdi swing with NAVA. EAdi peak and swing are indicators of diaphragmatic unloading, and are surrogates for WOB.10 We did not demonstrate a similar improvement in EAdi peak or swing with NAVA NIV in our trial. There was high variability in the EAdi measures obtained, which may have contributed to the lack of difference in the 2 groups. Perhaps, if measured longer or in more infants, a difference could be detected.
, peak inspiratory pressure, transcutaneous CO2, and EAdi peak.6,12,22,23 These studies, however, were of subjects who were intubated. Lee et al10 performed a study in a similar population by using a noninvasive interface and showed improved patient-ventilator synchrony and lower EAdi peak and EAdi swing with NAVA. EAdi peak and swing are indicators of diaphragmatic unloading, and are surrogates for WOB.10 We did not demonstrate a similar improvement in EAdi peak or swing with NAVA NIV in our trial. There was high variability in the EAdi measures obtained, which may have contributed to the lack of difference in the 2 groups. Perhaps, if measured longer or in more infants, a difference could be detected.
Lee et al10 used binasal prongs or nasal masks as the interface for infants in both modes of support, and a pneumatic trigger was used to synchronize ventilation in the pressure-support mode. This study found fewer asynchrony events, shorter trigger delay, and lower peak inspiratory pressures with NAVA NIV compared with noninvasive pressure-support ventilation in 15 neonates at <32 weeks’ gestation.
Beck et al9 in Canada showed feasibility and preservation of synchrony during NAVA NIV when they studied 5 very low birthweight neonates when using invasive NAVA, and then NAVA NIV delivered through a single nasal prong. Gibu et al11 also studied NAVA NIV in neonates by using neonatal CPAP prongs in a crossover study of 8 neonates comparing NAVA NIV to NIV. They found decreased peak inspiratory pressure and  with the use of NAVA NIV.11 To our knowledge, our study was the first study that used NAVA NIV in preterm neonates with a common nasal cannula interface used off-label (RAM cannula). Aside from improved patient-ventilator synchrony, we were unable to demonstrate other reported beneficial effects of NAVA NIV with this interface, perhaps due to its high resistance or questionable efficacy for delivering tidal volumes in NIV.19,24
with the use of NAVA NIV.11 To our knowledge, our study was the first study that used NAVA NIV in preterm neonates with a common nasal cannula interface used off-label (RAM cannula). Aside from improved patient-ventilator synchrony, we were unable to demonstrate other reported beneficial effects of NAVA NIV with this interface, perhaps due to its high resistance or questionable efficacy for delivering tidal volumes in NIV.19,24
Longhini et al25 evaluated differences in gas exchange, infant-ventilator interactions, respiratory drive, breathing pattern, vital signs, and sedation requirement during invasive and noninvasive NAVA in term infants. For the 10 infants studied, the parameters and synchrony were similar before and after extubation, which confirmed previous findings that patient-ventilator synchrony is preserved with NAVA with invasive and noninvasive interfaces. By using a Graseby capsul (Graseby Medical, UK) to attempt to synchronize NIV, Chang et al3 compared short-term effects of synchronized NIV with unsynchronized NIV and nasal CPAP in premature infants. Although they did not find differences in other respiratory parameters, by measuring esophageal pressure, they were able to show decreased WOB during synchronized NIV. Huang et al26 also used a Graseby capsule and compared synchronized with unsynchronized NIV in a crossover study of preterm infants who were recently extubated. They were able to show improved gas exchange and decreased respiratory effort during synchronized support.
When studying a largely unsynchronized mode of noninvasive pressure-support ventilation, Ali et al27 were able to show a decrease in indices of WOB, including thoracoabdominal asynchrony with the use of noninvasive pressure-support ventilation when compared with nasal CPAP. They did not find a change in minute ventilation. In our study, one unexpected finding involved the large number of intermittent backup breaths delivered to infants in the NAVA NIV mode. We suspected that these breaths were being delivered due to the short apnea times used in the study settings.
Apnea time is nomenclature used to define the time elapsed until a backup breath commences. Contrary to our previous understanding, we confirmed with the manufacturer that the apnea time as defined on the Servo-i ventilator measures the time to deliver a breath from the beginning of the most recent EAdi breath, not the end. For a breath cycle that could last 2 s in an infant breathing at a frequency of 30 breaths/min, this produced a large number of asynchronous backup breaths in infants in whom 2 s from the end of the last breath had not yet elapsed. For this reason, we recommend setting the apnea time with recognition of the infant’s usual breathing pattern.
Study Limitations
This physiologic trial was done during a limited time period at a single center in a small number of infants. Effects from the mode of ventilation that are only seen after prolonged ventilation using that mode could not be detected. We used a single noninvasive interface, the RAM cannula, which, although commonly used, is not ideal for provision of CPAP, NIV, or, apparently, NAVA NIV. During the NIV portion of the study, the ventilator rates used were higher and inspiratory times were shorter than some centers are accustomed to using. These settings may have contributed to asynchrony during this portion of the study. The suggestion that longer inspiratory times and slower rates improve synchrony is encountered in some recommendations, but data to support this hypothesis are limited.28 As found in survey data, many centers are using settings similar to those used in this study.29,30
We did not insert an esophageal balloon to measure transpulmonary pressure and calculate actual WOB because this would have been too invasive in this vulnerable population. An esophageal balloon is more invasive than the EAdi catheter, which is quite similar to a standard oro/nasogastric tube. We did, however, measure WOB in 2 ways, by using the phase angle and the EAdi, and found similar results with both methods. It is possible that there was a reduction in WOB that our methods did not detect.
Conclusions
NAVA NIV produces a marked improvement in patient-ventilator synchrony compared with NIV. We demonstrated no difference in WOB when compared with NIV. Other measured outcomes were also not different. Attention to apnea time and evaluation to determine the optimal noninvasive nasal interface is needed.
Footnotes
- Correspondence: David N Matlock MD, University of Arkansas for Medical Sciences, 4301 W. Markham St., Slot 512–5B, Little Rock, AR 72205. E-mail: DMatlock{at}uams.edu
Dr Matlock presented a version of this paper at the Society for Pediatric Research meeting held May 5–8, 2018 in Toronto, Canada.
Drs Beck and Sinderby and Mr Comtois have made inventions related to neural control of mechanical ventilation that are patented. Future commercial uses of this technology may provide financial benefit to Drs Beck and Sinderby through royalties. Drs Beck and Sinderby each own 50% of Neurovent Research Inc. The remaining authors have disclosed no conflicts of interest.
This research was supported by a grant from the Arkansas Children’s Research Institute.
- Copyright © 2020 by Daedalus Enterprises













