Abstract
BACKGROUND: Prone positioning (PP) during invasive mechanical ventilation improves outcomes of patients with severe ARDS. Recent studies suggest that PP in spontaneously breathing, nonintubated patients with acute respiratory failure is well tolerated and improves oxygenation. However, little is known regarding patient triggered ventilation in intubated patients with ARDS undergoing PP. We conducted a retrospective review of our experience with placing patients in the prone position in 2 cohorts of subjects with moderate and severe ARDS (ie, one cohort with ARDS related to COVID-19, the other with ARDS unrelated to COVID-19), many of whom were receiving pressure support ventilation (PSV).
METHODS: We conducted a retrospective analysis in a single 22-bed mixed ICU. The subjects included in the analysis were ≥ 18 y old, met the Berlin definition for moderate or severe ARDS (whether related COVID-19 or not), and underwent PP during invasive ventilation.
RESULTS: 39 subjects were included in the analysis: 20 subjects had ARDS related to COVID-19, while 19 had ARDS related to other etiologies. A total of 113 PP episodes were analyzed: 84 during PSV and 29 during volume control continuous mandatory ventilation. PP during PSV was well tolerated and was effective in improving arterial oxygenation (ie, an increase of median 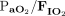 from 100 mm Hg [interquartile range 75–120] before PP to 135 mm Hg [interquartile range 111–161] at the end of the PP session, P < .0001). No significant difference between continuous mandatory ventilation and PSV was noted regarding arterial oxygenation during PP. Compared with continuous mandatory ventilation mode, PP during PSV was associated with a significant decrease in the use of neuromuscular blocking agents (4% vs 69% of subjects, P < .001), while sedative requirements remained unchanged.
from 100 mm Hg [interquartile range 75–120] before PP to 135 mm Hg [interquartile range 111–161] at the end of the PP session, P < .0001). No significant difference between continuous mandatory ventilation and PSV was noted regarding arterial oxygenation during PP. Compared with continuous mandatory ventilation mode, PP during PSV was associated with a significant decrease in the use of neuromuscular blocking agents (4% vs 69% of subjects, P < .001), while sedative requirements remained unchanged.
CONCLUSIONS: In a retrospective analysis of consecutive intubated subjects with moderate or severe ARDS, related or not to COVID-19, spontaneous breathing during PP was well tolerated and achieved significant improvement in arterial oxygenation.
Introduction
ARDS is common in critically ill patients, and it is associated with high mortality and morbidity.1 Prone positioning (PP) during invasive mechanical ventilation has been shown to improve oxygenation and to decrease mortality of the most severe cases of ARDS.2 Therefore, prone positioning for > 12 h/d is recommended in patients with severe ARDS.3 Although the benefits of prone positioning are well established, the ideal ventilatory management of patients with moderate and severe ARDS is less well defined. In particular, the benefits and risks of spontaneous breathing in patients with ARDS are debated. Experimental evidence is conflicting, and no clinical trial to date has compared assisted with volume control controlled mechanical ventilation.
Several studies, mainly retrospective, have suggested that prone positioning is safe and could improve oxygenation in spontaneously breathing, nonintubated patients with respiratory failure of various origins, some of them fulfilling ARDS criteria,4–6 and, recently, in subjects with severe hypoxemia due to coronavirus disease 2019 (COVID-19).7–9 However, none of the clinical studies that focused on the effects of prone positioning during invasive mechanical ventilation were conducted in subjects under a patient triggered ventilation mode. Therefore, we aimed to evaluate the feasibility, tolerance, and effects on oxygenation of prone positioning during moderate to severe ARDS, related to COVID-19 or not, in patients receiving pressure support ventilation (PSV). In our group, we tend to favor spontaneous breathing whenever possible in mechanically ventilated patients as part of a global strategy to decrease sedation requirements, promote early mobilization, and prevent muscular atrophy, including diaphragm dysfunction.10–13 In this regard, we try to maintain spontaneous breathing, most often in the PSV mode, while patients with moderate or severe ARDS undergo prone positioning. We conducted a retrospective review of our experience with placing 2 clinical series of subjects with moderate and severe ARDS in the prone position, many of whom were receiving PSV.
Quick look
Current Knowledge
Prone positioning during invasive ventilation improves outcomes of patients with severe ARDS. Recent studies suggest that prone positioning in spontaneously breathing, nonintubated patients with acute respiratory failure is well tolerated and improves oxygenation. No study has been conducted in spontaneously breathing, intubated patients, undergoing prone positioning for ARDS.
What This Paper Contributes to Our Knowledge
This retrospective analysis of consecutive subjects with moderate or severe ARDS, regardless of etiology, indicates that prone positioning during pressure support ventilation was well tolerated and effective in improving oxygenation.
Methods
The ethics committee at Comité d'Ethique Hospitalo-Facultaire, Saint-Luc (N°2019/16JAN/022 and N°2020/27AVR/247) approved the reporting of our study results. The need for informed consent was waived due to the retrospective nature of the analysis.
Subjects
We reviewed medical files for patients admitted from July 2012 to March 2018 in a 22-bed tertiary care mixed ICU to identify subjects for possible inclusion in this study. We searched for patients with a discharge diagnosis code of 518.50 (acute respiratory distress syndrome) and/or A03.5 (ARDS) according to the International Classification of Diseases, 9th Revision (ICD-9). The inclusion criteria were age ≥ 18 y at the time of diagnosis, findings consistent with the Berlin ARDS definition of moderate or severe ARDS,14 and prone positioning for > 12 h during the first 72 h of invasive mechanical ventilation. All consecutive patients who met inclusion criteria were included in the first cohort. A second cohort included all consecutive patients invasively ventilated for ARDS related to COVID-19 who underwent at least one session of prone positioning (for > 12 h) between March 15, 2020, to April 15, 2020.
Data Collection
Data were retrieved from the electronic medical record. Demographic data, underlying conditions, adjuvant therapies, and cause of ARDS were recorded. Acute Physiology and Chronic Health Evaluation II (APACHE II) score and Sequential Organ Failure Assessment (SOFA) score were calculated on the day of ARDS diagnosis. For all subjects, the first 4 sessions of prone positioning following initiation of mechanical ventilation were carefully considered. For each PP session, the occurrence of adverse events, respiratory and hemodynamic parameters (including vasoactive drugs use and dosing), and sedation-related variables (ie, Richmond Agitation and Sedation Scale, and the use and average dose of sedative drugs) were recorded. The following were considered adverse events: cardiac arrest, unplanned extubation, and main bronchus intubation due to endotracheal tube displacement. Hemodynamic instability was defined as bradycardia (< 30 beats/min for > 1 min), a decrease of mean arterial pressure of ≥ 20 mm Hg, or an increase of norepinephrine dose of 0.1 μg/kg/min in the 15 min following turning to prone position. We assessed each variable retrieved from the electronic medical record at 4 time points: immediately before PP, at the beginning of PP (first available values 1 h after initiation of PP for respiratory variables and values 15 min after initiation of PP for hemodynamic variables), at the end of PP (last value before turning back to supine position), and after PP (first available values > 1 h after turning back to supine position). For each session of PP, the duration of spontaneous breathing under PSV was recorded. When the duration of PSV exceeded 50% of the total duration of PP, the PP session was recorded in the PSV group; otherwise the session was recorded in the volume control continuous mandatory ventilation (VC-CMV) group. Subjects were followed until hospital discharge, and outcomes were recorded at 28 d and at ICU discharge. The detailed protocol used for prone positioning can be found in the supplementary materials (see the supplementary materials at http://www.rcjournal.com).
Statistical Analysis
Statistical analyses were performed using SPSS 21 (IBM, Armonk, New York), with graphs drawn using Graphpad Prism 8 (GraphPad Software, La Jolla, California). Values were expressed as median (interquartile range [IQR]) or mean ± SD for continuous values and count (percentage) for qualitative variables. The data were subjected to the Kolmogorov-Smirnov normality test and the Bartlett test for homogeneity of variance. We compared outcome and demographic variables, sedation, and adverse events between subject groups with the chi-square test (or Fisher exact test when appropriate) and the unpaired t test (or the Mann-Whitney test according to statistical distribution) for quantitative data. Furthermore, we compared the evolution of respiratory and hemodynamic parameters during PP sessions within each group using the paired t test (or Wilcoxon rank-sum test according to statistical distribution). All tests were 2-sided, with significance level set at .05.
Results
From July 2012 to March 2018, 107 subjects were admitted to the ICU with moderate or severe ARDS and underwent mechanical ventilation for ≥ 72 h. Thirty-eight subjects underwent PP, but full data (mainly regarding sedation and hemodynamic parameters) were available in only 19 subjects; these subjects were included in the first cohort (Fig. 1). From March 15 to April 15, 2020, 52 patients with severe COVID-19 were admitted to the ICU. Among them, 23 underwent invasive ventilation and 20 underwent ≥ 12 h of PP. Full data were available for all subjects who were included in the second cohort. The baseline characteristics, cause of ARDS, and main outcome data for 39 subjects with mild and moderate ARDS who were mechanically ventilated and underwent prone positioning for ARDS unrelated to COVID-19 (first cohort) or for ARDS related to COVID-19 (second cohort) are summarized in Table 1. The median age of the cohort was 59 y. Subjects undergoing PP for ARDS related to COVID-19 were more likely to be male (65% vs 37%, P = .07) and had lower APACHE II scores (11 [IQR 9–21] vs 25 [IQR 18–28], P = .001) and lower SOFA score (5 [IQR 4–9] vs 9 [IQR 5–12], P = .01). The 28-d and ICU mortality rates were 31% and 42% in the whole cohort, respectively, with no difference between subjects with ARDS related or unrelated to COVID-19. There were 113 sessions of PP, with more PP sessions in PSV (84) than in VC-CMV (29). Thirty-four subjects (87%) experienced ≥ 1 session of PP under PSV. Subjects with COVID-19 had significantly more PP sessions (4 vs 2, P = .02) and were less likely to have PP during PSV (66% vs 87% of PP sessions, P = .01).
Flow chart.
Baseline Characteristics, Respiratory Parameters, and Outcomes
Baseline characteristics and outcomes of subjects classified into a full PSV group (ie, all PP sessions during PSV, n = 25), a full control group (ie, all PP sessions during VC-CMV, n = 5), or in a combination group (ie, PP sessions during either PSV or VC-CMV, n = 9) are shown in the supplementary materials (see the supplementary materials at http://www.rcjournal.com).
As shown in Table 2, PP duration was significantly shorter in the PSV group (14.6 vs 17 h, P = .02). There was no difference regarding the number of sedative drugs nor the average dose during PP between VC-CMV and PSV. Use of neuromuscular blocking agents (NMBAs) was more frequent in the VC-CMV group than in the PSV group (69% vs 4% of sessions, P < .001). Four sessions of PP, all in the PSV group, were terminated prematurely due to a severe adverse event (1 cardiac arrest, 2 unplanned extubations, and 1 main bronchus intubation during PP procedure).
Comparison Between Sedative Use and Adverse Events With PP in Different Ventilator Modes
The evolution of respiratory parameters before, during, and after prone positioning is shown in Figure 2 and detailed in Table 3. As expected,  increased with PP. Other respiratory parameters remained unchanged throughout PP sessions, other than a significant decrease in median breathing frequency from 30 breaths/min (IQR 22–36) to 26 breaths/min (IQR 21–32) in the PSV group (P = .03). The detailed evolution of
increased with PP. Other respiratory parameters remained unchanged throughout PP sessions, other than a significant decrease in median breathing frequency from 30 breaths/min (IQR 22–36) to 26 breaths/min (IQR 21–32) in the PSV group (P = .03). The detailed evolution of  throughout all 4 PP sessions in the VC-CMV group and the PSV group is provided in the supplementary materials (see the supplementary materials at http://www.rcjournal.com).
throughout all 4 PP sessions in the VC-CMV group and the PSV group is provided in the supplementary materials (see the supplementary materials at http://www.rcjournal.com).
Evolution of respiratory parameters * P < .05, ** P < .005, *** P < .0005 throughout prone position sessions in pressure support ventilation and in volume control continuous mandatory ventilation. PSV = pressure support ventilation, VC-CMV = volume control continuous mandatory ventilation, VT = tidal volume, PBW=predicted body weight.
Evolution of Respiratory Parameters Before, During, and After Prone Positioning
The evolution of hemodynamic parameters during PP sessions is shown in the supplementary materials (see the supplementary materials at http://www.rcjournal.com). We did not find any significant change in mean arterial pressure, heart rate, or norepinephrin dose during PP in either the PSV group or the VC-CMV group.
Discussion
To our knowledge, this study is the first to focus on PP in the early phase of moderate and severe ARDS, whether related to COVID-19 or not, in subjects spontaneously breathing under invasive mechanical ventilation. In our work, we describe the early application of PP in 39 subjects with moderate to severe ARDS, among whom 34 experienced ≥ 1 session of PP in PSV. A total of 84 PP procedures were performed during PSV and were compared with 29 PP procedures performed during VC-CMV. Our findings indicate that PP during PSV was effective in improving arterial oxygenation, well tolerated, and associated with a significant decrease in NMBA consumption compared with PP during VC-CMV, without change in sedative requirements.
Few interventions have demonstrated a survival benefit in patients with moderate or severe ARDS. Among them, prone positioning is an inexpensive and well-tolerated intervention whose benefits for the outcomes of patients with moderate or severe ARDS have been demonstrated in recent trials.2,15–17 Prone positioning improves oxygenation by optimizing lung recruitment and ventilation-perfusion matching, helps prevent ventilator-induced lung injury by improving the distribution of mechanical forces through the lung, and encourages secretion drainage.18 Despite these recognized benefits, PP is still underused.1,15 A recent prevalence study reported that PP was used in only 33% of subjects with severe ARDS.15 The main reasons given for not using PP in subjects with severe ARDS were the rating of hypoxemia as “not severe enough” and hemodynamic instability.15
In our study, PP was well tolerated, with a rate of severe complications of 3%, lower than previously reported in clinical trials2,17 and comparable to the rate of complications recorded in a recent prevalence study.15 One subject had a cardiac arrest during PP, but it happened > 4 h after being turned to the prone position; the subject ultimately survived. Transient hemodynamic instability at the initiation of PP was frequent (26% of subjects) but limited in duration. In addition, norepinephrine doses remained stable throughout the PP procedures. Our results indicate that, with an experienced team, PP can be safely managed even while patients are spontaneously breathing under mechanical ventilation.
Maintaining PSV during PP procedures led, unsurprisingly, to decreased NMBA use, with no differences regarding sedatives; transient muscular paralysis for turning to the prone position was used for a few subjects, explaining the 4% of PP procedures with NMBA use in the PSV group. The use of NMBA in ARDS is still debated. Whereas the ACURASYS trial reported a decrease in mortality in subjects with severe ARDS treated with cisatracurium for 48 h,19 those results were not replicated in a recent larger trial.20 Moreover, prolonged NMBA use is discouraged21 because of their potential to contribute to sustained neuromuscular weakness,22 ventilator-associated diaphragm dysfunction,23,24 and impaired coughing and secretion clearance. A significant proportion of patients with ARDS, particularly in the case of ARDS related to COVID-19, experience severe hypoxemia for prolonged periods and might benefit from multiple sessions of PP, with potential exposure to the deleterious effects of long-term neuromuscular blockade. Moreover, due to the recent pandemic of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), ICUs across the world are faced with a dramatic increase of patients with severe ARDS. In many areas, supplies of critical care medications, including NMBAs, are extremely limited, with expected or effective shortages.25 For those reasons, utilizing PP procedures under PSV without the use of NMBA might prove useful.
Furthermore, we showed that PP performed during PSV was effective in improving arterial oxygenation. The increase of  in our cohort was not different between PSV and VC-CMV group. Interestingly, in subjects undergoing PP under PSV, there was a significant decrease in breathing frequency with no change in tidal volume, suggesting a reduced work of breathing.
in our cohort was not different between PSV and VC-CMV group. Interestingly, in subjects undergoing PP under PSV, there was a significant decrease in breathing frequency with no change in tidal volume, suggesting a reduced work of breathing.
Beyond the effectiveness of PP to improve oxygenation in invasively ventilated patients with ARDS, the question of the use of spontaneous ventilation in ARDS remains open. Experimental evidence is conflicting and clinical evidence is scarce. Therefore, it is still unknown whether the potential benefits of spontaneous breathing (eg, better patient-ventilator interaction, prevention of diaphragmatic atrophy and atelectasis, better ventilation of dorsal lung units, decreased sedation requirements13,26–28) outpace the harms (eg, higher rate of respiratory asynchronies, increase in transpulmonary pressure and pendelluft phenomenon26,29) in a given patient. Despite this uncertainty, it was reported in a secondary analysis of the LUNG SAFE database that spontaneous breathing was common in the first 48 h after ARDS diagnosis, with 79% and 21% of subjects exhibiting transient or continuous spontaneous breathing, respectively.30 In an adjusted analysis, spontaneous breathing was not associated with deleterious outcomes.
Our study was not designed or powered to evaluate the effects of spontaneous breathing on outcomes. However, the ICU mortality rates in subjects with ARDS unrelated to COVID-19 or with ARDS related to COVID-19 (42% in both cohorts) were similar to those reported in recent large series in which subjects were managed with conventional ventilation strategies.1,31,32
Our results should be interpreted with caution. First, it is a retrospective analysis of a small number of subjects in a single referral center. Second, in our analysis, we pooled subjects with ARDS secondary to COVID-19 and subjects with ARDS from other etiologies, despite the fact that the underlying pathophysiology might be different.33 It has been suggested that only a proportion of patients with COVID-19 pneumonia can be qualified as ARDS because the respiratory system compliance remains initially normal in a large proportion of these patients.33,34 According to this hypothesis, the expected benefits in the 2 subpopulations of subjects with ARDS may be related to different physiopathological phenomena and should be analyzed separately. However, a recent prospective multicenter study reported that patients with ARDS related to COVID-19 have lung morphology and respiratory mechanics that match those of subjects with ARDS unrelated to COVID-19.35 Moreover, we observed comparable results when we analyzed the effects of PP within each of the 2 cohorts. Third, there was an imbalance between subjects in the PSV mode and subjects in the VC-CMV mode, with a large majority in the former. Our habit is to favor spontaneous breathing whenever possible. The use of VC-CMV is usually restricted to asynchronies or poor tolerance during PSV, or to large tidal volumes despite adaptation of ventilator settings. This could constitute a selection bias toward the use of VC-CMV in patients with more severe ARDS. However,  was similar between groups. Fourth, in the first cohort, only 35% of all subjects with moderate to severe ARDS underwent PP. One possible explanation for this “underuse” of PP is that a careful optimization of ventilator parameters, combined with adequate sedation, can achieve significant improvement in oxygenation in many subjects and could therefore make PP unnecessary. In our usual practice, we reserve PP for patients who remain severely hypoxemic (
was similar between groups. Fourth, in the first cohort, only 35% of all subjects with moderate to severe ARDS underwent PP. One possible explanation for this “underuse” of PP is that a careful optimization of ventilator parameters, combined with adequate sedation, can achieve significant improvement in oxygenation in many subjects and could therefore make PP unnecessary. In our usual practice, we reserve PP for patients who remain severely hypoxemic ( < 150 mm Hg) despite adequate ventilator optimization. Moreover, an equally low prevalence of PP among patients with severe ARDS was reported in the APRONET study.15 Lastly, data were missing for many of the patients with ARDS unrelated to COVID-19. These patients were not included in the analysis, which constitutes another possible selection bias.
< 150 mm Hg) despite adequate ventilator optimization. Moreover, an equally low prevalence of PP among patients with severe ARDS was reported in the APRONET study.15 Lastly, data were missing for many of the patients with ARDS unrelated to COVID-19. These patients were not included in the analysis, which constitutes another possible selection bias.
Conclusions
In this retrospective analysis of consecutive subjects with moderate or severe ARDS, whether related to COVID-19 or not, PP during PSV was well tolerated and effective in improving oxygenation. When compared with controlled mechanical ventilation (ie, CMV), PP procedures during PSV were associated with a significant decrease in NMBA consumption, whereas sedative requirements remained unchanged. Large, prospective, randomized controlled trials are needed to compare assisted to controlled mechanical ventilation in patients with ARDS, particularly those who undergo prone positioning. Moreover, physiologic studies focusing on the effects of spontaneous breathing during prone positioning should help identify patients in whom the benefits of spontaneous breathing are likely to outweigh potential harm.
Acknowledgments
We thank P. Delrez for his assistance in collecting the data used for this work. We thank the ICU physicians and nurses who took care of our patients during the COVID-19 pandemic.
Footnotes
- Correspondence: Ludovic Gerard MD, Université Catholique de Louvain, Cliniques Universitaires Saint-Luc, Department of Critical Care Medicine, Avenue Hippocrate 10, B-1200 Bruxelles, Belgium. E-mail: ludovic.gerard{at}uclouvain.be
Supplementary material related to this paper is available at http://www.rcjournal.com.
The authors have disclosed no conflicts of interest.
See the Related Editorial on Page 884
- Copyright © 2021 by Daedalus Enterprises













