Abstract
BACKGROUND: Suspensions delivered via a pressurized metered-dose inhaler (pMDI) require shaking the canister before actuation to prevent drug sedimentation. We hypothesized that a shake-actuation delay of an albuterol hydrofluoroalkane (HFA) pMDI will increase and decrease delivered dose (DOSE) at the beginning and end of the canister’s life, respectively, and that aerosol characteristics will remain unchanged with the delay.
METHODS: Albuterol HFA pMDIs (90 µg/actuation, 200 doses) operated with and without a 30-s shake-actuation delay, and with and without a valved holding chamber (VHC), were studied. Ten puffs, with a 30-s interval between puffs and 5s of shaking, were actuated into a drug-recovery apparatus. Inhalers were studied throughout their entire life (from 200 doses to 0 remaining doses). Particle size analysis was performed with cascade impaction at the beginning, middle, and end of the canister’s life. Albuterol mass was determined via spectrophotometry (276 nm).
RESULTS: Mean (99% CI) delivered dose without shake-actuation delay was 100.4% (98.1–102.9%) of the nominal dose. Mean (99% CI) delivered dose with a VHC without a shake-actuation delay was 48.6% (46.2–50.9%) of the nominal dose. The shake-actuation delay increased and decreased delivered dose at the beginning and end of the canister’s life, respectively, irrespective of VHC use. Decline in delivered dose began when 110–120 doses remained in the canister. Aerosol characteristics remained constant throughout the canister’s life except for the amount of drug captured by the impactor for the pMDI with delay and the pMDI/VHC with delay. Adding a VHC to a pMDI operated with a delay increased fine-particle fraction and decreased the amount of drug captured by the impactor at the middle and at the end of the canister’s life.
CONCLUSIONS: A 30-s shake-actuation delay of an albuterol HFA pMDI increased and decreased delivered dose at the beginning and end of canister’s life, respectively. Particle size characteristics at the end of the canister’s life changed when the pMDI and pMDI/VHC were operated with a shake-actuation delay. Patients should re-shake the pMDI if it is not actuated immediately after shaking the canister.
Introduction
Asthma is a serious global health problem affecting all age groups, with global prevalence ranging from 1% to 21% in adults, and with up to 20% of children 6–7 y old experiencing severe wheezing episodes within a year.1 Bronchodilators such as albuterol are used to treat acute asthma exacerbations. Albuterol is available as a nebulizer solution, as a dry powder inhaler, and as a suspension in a pressurized metered-dose inhaler (pMDI). The latter is widely used due to its portability. Valved-holding chambers (VHCs) are used in pediatric and geriatric population to overcome the challenges of coordinating actuation and inhalation. It is common that a delay between shaking and actuation of the canister occurs in these populations.
Proper operation of the pMDI is paramount for optimal drug delivery. The use of drugs formulated as suspensions require shaking of the canister before actuation to reverse the sedimentation of the drug that occurs when propellant and drug have different densities. Ex vivo and in vitro studies have reported that a delay between shaking and actuation causes a change in the amount of drug released by the pMDI.2-5 However, these studies have some limitations, including being done at the beginning of the canister’s life,2 using only 1 inhaler,4 using several delay times with the same inhaler,3,5 not using a VHC, and not measuring particle size. It is unknown whether the increase in delivered dose observed at the beginning of the canister’s life could result in changes of the aerosol characteristics due to agglomeration of the particles. This would result in a larger particle size emitted at the beginning of the canister’s life compared to the aerosol emitted at the end of the canister’s life.
Investigating the effect on drug delivery of a delay between shaking and actuation of an albuterol pMDI suspension with and without the use of a VHC is clinically relevant. This scenario occurs when the inhaler is shaken and the caregiver is trying to give the treatment to an uncooperative patient. Although we did not anticipate a different outcome when the VHC was used, we considered that including VHC testing was relevant given its widespread use. On the basis of previous studies, despite their limitations, we hypothesized that introducing a delay between the shaking and actuation of the pMDI, with or without a VHC, will cause an increase in the delivered dose at the beginning of the canister’s life and a decrease in the delivered dose at the end of the canister’s life. We also hypothesized that aerosol characteristics will not vary throughout the canister’s life (null hypothesis).
Quick Look
Current Knowledge
Proper use of pMDIs is paramount for effective drug delivery. Patients are instructed to shake the canister of pMDI albuterol before each actuation. Sometimes a delay occurs between shaking and actuation of the pMDI. This is common in pediatric and uncooperative patients.
What This Paper Contributes to Our Knowledge
A 30-s delay between shaking and actuation resulted in an increase and decrease of delivered dose at the beginning and end of the canister’s life, respectively. Decline in delivered dose began when 110–120 doses remained in the canister. Patients and parents should be instructed to shake the canister again if it is not immediately actuated to avoid potential under- or overdosing.
Methods
The experiments were performed at the Pediatric Aerosol Research Laboratory at Arkansas Children’s Research Institute in Little Rock, Arkansas. The study included 4 scenarios: pMDI alone without delay, pMDI used with VHC (pMDI/VHC) without delay, pMDI alone with delay, and pMDI/VHC with delay. Delivered dose was studied for all scenarios, and particle size characterization was done for all scenarios except for pMDI/VHC without delay.
Procedure for Measuring Delivered Dose
A total of 12 new canisters (3 for each scenario), from the same lot, of albuterol sulfate 90 μg/actuation (Ventolin hydrofluoroalkane [HFA], 200 actuations; GlaxoSmithKline, Philadelphia, Pennsylvania) were used. Canisters were primed with 4 puffs per manufacturer’s instructions. The inhaler was shaken for 5 s and then actuated into a recovery apparatus (Fig. 1A). This maneuver was performed 10 times with a 30-s interval between each actuation.6 This was done in sets of 10 puffs throughout the canister’s life (from 200 to 0 actuations) except for the actuation ranges of 190–180, 100–90, and 20–10 (Table 1). At these 3 actuation ranges, particle size characterization was performed with a Next Generation Impactor (NGI model 170, MSP Corporation, Shoreview, Minnesota).7 We used 10 puffs to ensure enough drug was present in the recovery apparatus and the NGI similarly to previous publications.6 In addition, the most current international guidelines recommend 4–10 puffs of a short-acting bronchodilator for the treatment of mild to moderate exacerbation.1
Recovery apparatus. A: Recovery apparatus alone; B: recovery apparatus with pMDI attached; and C: recovery apparatus with pMDI and valved holding chamber attached.
Testing Schedule
The recovery apparatus consisted of a low dead-space volume filter holder with a disposable filter media (Pari Respiratory Equipment, Midlothian, Virginia) and an elbow followed by an adapter. The apparatus (internal volume of 43 mL) was connected at one end to a vacuum pump (HCP 5, MSP Corporation) and at the other end to the mouthpiece of either the pMDI or the VHC. The vacuum pump connected to the recovery apparatus was calibrated at 30 L/min before each procedure with a mass flow meter (model 4043, TSI, Shoreview, Minnesota). Temperature and humidity were recorded before each procedure. Canister weight as well as puff number were recorded before and after every procedure for quality control. Upon completion of each set of 10 puffs, albuterol was eluted from the filter and the elbow/connector with double-distilled water, and drug concentration was measured with spectrophotometry at 276 nm (Smart Spec Spectrometer, Bio-Rad Laboratories, Hercules, California).
These experiments were repeated with a 30-s delay between shaking and actuation, as well as with the addition of a VHC (Optichamber Diamond, Philips Respironics, Chichester, United Kingdom) with and without delay (Fig. 1B, C).
The outcome variable for these experiment was the amount of drug captured in the recovery apparatus (DOSE) for pMDI alone without delay (DOSEa), for pMDI alone operated with delay (DOSEa-delay), for pMDI/VHC without delay (DOSEvhc), and for pMDI/VHC operated with delay (DOSEvhc-delay).
Particle Size Characterization
An NGI assembled with internal and external filters was used to determine particle size of the emitted aerosol at the beginning, middle, and end of canister’s life (at puff ranges of 190–180, 100–90, and 20–10, respectively). At the beginning of each procedure, temperature and humidity were documented, and the impactor was calibrated to 30 L/min using the same mass flow meter. A silicon adapter was used to connect the induction port of the NGI to the mouthpiece of either the pMDI or the VHC (Fig. 2). The impactor was powered on, and the pMDI was shaken for 5 s and then connected to the adapter and actuated once. This procedure was performed 10 times with a 30-s interval between actuations. The canisters were weighed at the beginning and at the end of the 10 actuations for quality control. The impactor was disassembled at the end of 10 actuations, and the induction port, cups, and filters were eluted with 10 mL double-distilled water. All washings were tested for albuterol concentration using spectrophotometry.
Particle size characterization setup. A: Next Generation Impactor connected to a pressurized metered-dose inhaler. B: Next Generation Impactor connected to valved holding chamber and a pressurized metered-dose inhaler.
The experiments were repeated with pMDI operated with a 30-s delay between shaking and actuation and with a pMDI/VHC operated with a 30-s delay between shaking and actuation.
Mass median aerodynamic diameter (MMAD), geometric standard deviation (GSD), fine-particle fraction (FPF; percentage of particles < 5 µm), 1–3 µm fraction (1-3F; the percentage of particles 1–3 µm in size), and total mass captured by the NGI (MASS) were calculated with CITDAS 3.1 software (Copley Scientific, Nottingham, United Kingdom).6
Statistical Analysis
DOSE was expressed as percentage of the nominal dose (900 µg). Mean (95% CI) of DOSEa were calculated and compared to DOSEa-delay at different intervals of the canister’s life (unpaired t test with unequal variance). The number of occasions that DOSEa-delay was outside the 95% CI of DOSEa and the number of occasions DOSEa-delay was either 20% higher or lower than DOSEa were calculated. Similar comparisons were done between DOSEvhc and DOSEvhc-delay. Comparison of aerosol characteristics across the life of the inhaler was done with analysis of variance for repeated measures followed by Tukey test when necessary. Comparisons between pMDI operated with and without delay, and between pMDI and pMDI/VHC operated with delay, were done with unpaired t test with unequal variances. A P value of .05 was considered statistically significant. Kaleidagraph 4.53 statistical software was used for all of the calculations (Synergy Software, Reading, Pennsylvania).
Results
Delivered Dose (DOSE)
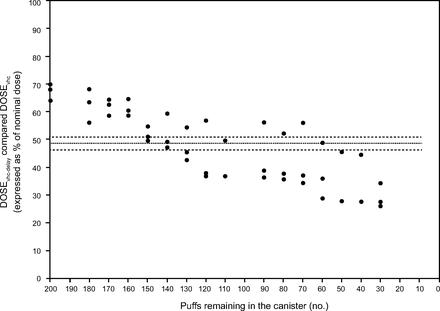
The mean (99% CI) canister weight change was 0.721 g/10 puffs (0.718–0.723 g/10 puffs). The mean (99% CI) for DOSEa was 100.4% (98.1–102.9%) of the nominal dose (Fig. 3). DOSEa-delay was above and below the 99% CI of DOSEa 41% and 41% of the times, respectively. A decline in DOSEa-delay began when 120 puffs remained in the canister. DOSEa-delay was 20% higher or lower than mean DOSEa 16% and 24% of the times, respectively. DOSEa was lower than DOSEa-delay for the first 60 puffs (P = .048), and higher than DOSEa-delay for the last 40 puffs (P = .03).
The dotted line represents the mean value of delivered dose (DOSE) of a pressurized metered-dose inhaler alone operated without and with delay (DOSEa). The dashed line represents the 95% CI around the mean DOSEa. Each circle represents an individual measurement of DOSEa delay.
The mean (99% CI) for DOSEvhc was 48.6% (46.2–50.9%) of the nominal dose (Fig. 4). DOSEvhc-delay was above and below the 99% CI of DOSEvhc 41% and 41% of the times, respectively. A decline in DOSEvhc-delay began when 110 puffs remained in the canister. DOSEvhc-delay was 20% higher or lower than mean DOSEvhc 29% and 47% of the times, respectively. DOSEvhc was lower than DOSEvhc-delay for the first 60 puffs (P = .03) but similar in the remaining 140 puffs. The use of a VHC resulted in a median (99% CI) DOSE reduction of 51.1% (55.6–47.8%) and 50.6% (54.8–47.4%) for the pMDI operated without and with delay, respectively.
The dotted line represents the mean value of delivered dose (DOSE) of a pressurized metered-dose inhaler operated with delay alone and attached to a valved holding chamber (DOSEvhc). The dashed line represents the 95% CI around the mean DOSEvhc. Each circle represents an individual measurement of DOSEvhc without delay.
Particle Size Characterization
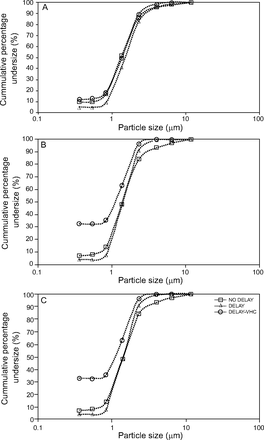
A summary of the particle size characterization obtained at different intervals of the canister’s life and under different conditions can be seen in Table 2. A plot of cumulative percentage undersize against particle size for pMDI alone, pMDI alone with delay, and pMDI/VHC with delay can be seen in Figure 5.
Cumulative percentage undersize against particle size of albuterol hydrofluoroalkane pressurized metered-dose inhaler (pMDI). (A) Puff interval 190–180; (B) puff interval 100–90; (C) puff interval 20–10. NO DELAY = pMDI alone without delay; DELAY = pMDI alone with delay; DELAY-VHC = pMDI/VHC with delay. VHC = valved holding chamber.
Particle Size Characteristics of Albuterol pMDI at Different Intervals of the Canister’s Life
Comparison Across Canister Life.
There was no difference in MMAD across the canister’s life for pMDI alone, pMDI alone operated with delay, and pMDI/VHC operated with delay (P = .38, P = .44, and P = .19, respectively). There was no difference in GSD across the canister’s life for pMDI alone, pMDI alone operated with delay, and pMDI/VHC operated with delay (P = .94, P = .22, and P = .14, respectively). There was no difference in FPF across the canister’s life for pMDI alone, pMDI alone operated with delay, and pMDI/VHC operated with delay (P = .63, P = .57, and P = .069, respectively). There was no difference in 1–3F across the canister’s life for pMDI alone and pMDI alone with delay (P = .42, P = .11, respectively). However, 1–3F was smaller at 190–180 puffs than at 100–90 or 20–10 puffs with pMDI/VHC operated with delay (P = .02 and P = .01, respectively). There was no difference in MASS across the canister’s life for pMDI alone and pMDI alone operated with delay (P = .42, P = .11, respectively). The pMDI/VHC operated with delay showed a progressive decline across the canister’s life (P < .001).
pMDI Without Delay versus pMDI With Delay.
There were no differences in MMAD, GSD, FPF, and 1–3F across similar intervals of the canister’s life between the pMDI operated without or with delay (P > .05 for all). There was no difference in MASS at the 190–180 and 100–90 puff intervals between the pMDI operated without or with delay (P = .20 and P = .48, respectively). However, pMDI operated without delay had higher MASS than pMDI operated with delay at the 20–10 puff interval (P = .001).
pMDI With Delay versus pMDI/VHC With Delay.
There were no differences in MMAD and GSD across similar intervals of the canister’s life between pMDI operated with delay and pMDI/VHC operated with delay (P > .05 for all). There were significant differences in FPF and 1–3F across similar intervals of the canister’s life between pMDI operated with delay and pMDI/VHC operated with delay (P > .05 for all) (Fig. 5). There was no difference in MASS at the 190–180 puff interval between pMDI operated with delay and pMDI/VHC operated with delay (P = .11). However, pMDI operated with delay had higher MASS than pMDI/VHC operated with delay at the 100–90 and 20–10 puff intervals (P = .032, P = .001, respectively).
Discussion
Introducing a 30-s shake-actuation delay when operating a pMDI of albuterol HFA resulted in an increase and decrease in DOSE at the beginning and end of canister’s life, respectively. This occurred when the pMDI was operated alone and when it was attached to a VHC. Decline in DOSE began when 110–120 doses remained in the canister. In addition, aerosol characteristics remained essentially unchanged except for MASS, which decreased with canister life with the pMDI/VHC operated with a delay.
The increase in DOSE at the beginning of the canister’s life is in agreement with previous studies done with albuterol HFA and fluticasone HFA.2,3,5 However, the magnitude of change in our study was smaller. The difference could be attributed to the fact the other studies used the same inhaler to test different delay times ranging from 0 s to 60 s. This could result in an overestimation of the effect. In addition, they used a different recovery apparatus but with the same flow.
Hatley et al4 reported similar results, namely an increase in DOSE at the beginning of the canister’s life that was followed by a progressive decrease. Their study compared no delay to a 10-s shake delay using only 1 canister for each scenario. They noted that the decline in DOSE started when 110 puffs remained in the canister, and that DOSE was 120%, 100%, and 60% of the nominal dose at the beginning, middle, and end of the canister’s life, respectively. These data mirror our results when using a 30-s delay. Hatley et al4 also looked at a 60-s delay, which resulted in a DOSE that was 300%, 50%, and 25% of the nominal dose at the beginning, middle, and end of the canister’s life, respectively. These results are consistent with the sedimentation process that occurs over time because albuterol’s density (1.30 g/mL) is greater than HFA’s density (1.22 g/mL).
None of the previous studies used a VHC when testing DOSE after a delay was introduced. This is clinically relevant because younger pediatric patients and uncooperative patients often use a VHC. Similar to using a pMDI alone, DOSE increased at the beginning and decreased toward the end of the canister’s life when a VHC was attached to a pMDI operated with a 30-s shake-actuation delay.
Another novelty of this study was the evaluation of particle size across the canister’s life. The particle size characteristics were similar to previous reports except for slightly different FPFs.6,8 Aerosol characteristics remained essentially constant across the canister’s life for each studied scenario (pMDI alone, pMDI alone with delay, pMDI/VHC with delay). One exception was an increase in 1–3F that was noted between the beginning and the end of the canister’s life. Although statistically significant, its magnitude is not clinically important. The only other exception was a progressive decrease in MASS from the beginning to the end of the canister’s life. This is consistent with our reported findings of DOSE.
The introduction of a shake-actuation delay did not change aerosol characteristics except at the end of the canister’s life when MASS was decreased. However, the addition of a VHC to a pMDI operated with a shake-actuation delay resulted in an expected increase in FPF and 1–3F and a decrease in MASS. The latter is due to the amount of drug retained inside the VHC. This is consistent with previous studies that compared aerosol characteristics of pMDI operated without delay with and without VHC.6 The decrease in MASS seen with the use of a VHC does not diminish its utility because its main benefit is to allow use of pMDI in patients who can’t coordinate actuation and inhalation. A discrepancy between DOSE and MASS was noted, with the latter being lower. We speculate that the difference is likely due to the recovery process. While DOSE is obtained from 2 sources (ie, filter and elbow), MASS is obtained from 10 sources (induction port, collections cups, and filters).
The plot of cumulative percentage undersize against particle size showed similar characteristics for pMDI alone with and without delay throughout the canister’s life (Fig. 5). A pMDI with a VHC operated with a delay had characteristics similar to others at the beginning of the canister’s life. However, it showed a non-zero start at the middle and end of the canister’s life. This may be due to the formulation containing a significant amount of small particles, or to larger particles bouncing and re-entering smaller size stages. The latter is the most likely cause and constitutes a limitation for the data for the pMDI/VHC with delay because we did not perform a validation study to rule this out.
The clinical implications are 2-fold. First, patients and practitioners who introduce a 30-s shake-actuation delay when using a pMDI alone or attached to a VHC will deliver a higher DOSE than desired if pMDI is used between the beginning and middle of the canister’s life. Conversely, a lower DOSE will be delivered if the pMDI is operated between the middle and the end of the canister’s life. Therefore, the introduction of a shake-actuation delay could potentially result in higher risk of adverse effects (eg, tachycardia) or treatment failure due to over- and underdosing, respectively. The latter is more significant if low doses are used. These results are relevant for younger patients for uncooperative patients of any age. These findings are more relevant now when common canister protocols for pMDIs are being used due to poor accessibility caused by the increase in use in pMDI during the coronavirus pandemic. The researchers recommend that practitioners providing inhaler training should advise their patients to re-shake the canister if it is not actuated immediately after shaking to avoid this problem. Moreover, manufacturers should consider adding this recommendation to their instructions for use.
Limitations of this study include the fact that the researchers tested groups of 10 puffs together rather than fewer puffs at a time. The average value for the 10 puffs collected does not necessarily mean that each of the individual puffs emitted the same dose. However, this was done to make sure that enough drug was available to be quantified and to resemble international recommendations.1 We studied the particle size characteristics at 3 points in the canister’s life. Finally, the researchers only evaluated a 30-s shake-actuation delay; however, we deemed the interval to be clinically relevant.
Conclusions
Using an albuterol HFA pMDI with a 30-s shake-actuation delay resulted in increased and decreased DOSE at the beginning and end of the canister’s life, respectively. Particle size characteristics at the end of the canister’s life changed when the pMDI and pMDI/VHC were operated with a shake-actuation delay. Patients should re-shake the pMDI if it is not actuated immediately after shaking the canister to avoid potential over- or underdosing.
Footnotes
- Correspondence: Ariel Berlinski MD FAARC, 1 Children’s Way, Slot 512–17, Little Rock, AR 72212. E-mail: berlinskiariel{at}uams.edu
Dr Qaqish presented a version of this paper at the 2020 American Thoracic Society Virtual Conference, held August 5 through November 10, 2020.
This work was partially supported by the James H. Hamlen II Endowed Chair in Pediatric Pulmonology funds (Arkansas Children’s Hospital). Dr Berlinski has disclosed relationships with AbbVie, Allergan, Anthera, DCI, Cempra, Cystic Fibrosis Foundation, National Institute of Health, Novartis, Therapeutic Development Network, Trudell Medical International, Vertex, Vivus, and the International Pharmaceutical Aerosol Consortium on Regulation and Science. Dr Qaqish has disclosed no conflicts of interest.
- Copyright © 2021 by Daedalus Enterprises