Abstract
BACKGROUND: Recent observational studies of nebulizers placed on the wet side of the humidifier suggest that, after some time, considerable condensation can form, which triggers an occlusion alarm. In the current study, an inline breath-enhanced jet nebulizer was tested and compared in vitro with a vibrating mesh nebulizer on the humidifier dry–inlet side of the ventilator circuit.
METHODS: Two duty cycle breathing patterns were tested during continuous infusion (5 or 10 mL/h) with and without dynamic changes in infusion flow and duty cycle, or bolus delivery (3 or 6 mL) of radiolabeled saline solution. Inhaled mass (IM) was measured by a real-time ratemeter (µCi/min) and analyzed by multiple linear regression.
RESULTS: During simple continuous infusion, IM increased linearly for both nebulizer types. IM variability was attributable to the duty cycle (P < .001) (34%) and infusion flow (P < .001) (32%) but independent of nebulizer technology (P = .38) (7%). Dynamic continuous infusion studies that simulate clinical scenarios with ventilator and pump flow changes demonstrated a linear increase in the rate of aerosol that was dependent on pump flow (P < .001) (63%) and minimally dependent on the duty cycle (P = .003) (8%). During bolus treatments, IM increased linearly to plateau. IM variability was attributable to the duty cycle (P < .001) (40%) and residual radioactivity in the nebulizer (P < .001) (20%). Separate analysis revealed that the vibrating mesh nebulizer residual volume contributed 16% of the variability and inline breath-enhanced jet nebulizer contributed 5%. IM variability was independent of bolus volume (P = .82) (1%). System losses were similar (the inline breath-enhanced jet nebulizer: 32% residual in nebulizer; the vibrating mesh nebulizer: 34% in circuitry).
CONCLUSIONS: Aerosol delivery during continuous infusion and bolus delivery was comparable between the inline breath-enhanced jet nebulizer and the vibrating mesh nebulizer, and was determined by pump flow and initial ventilator settings. Further adjustments in ventilator settings did not significantly affect drug delivery. Expiratory losses predicted by the duty cycle were reduced with placement of the nebulizer near the ventilator outlet.
Introduction
In vivo drug delivery to mechanically ventilated patients can be predicted in bench studies by measuring deposition on a filter positioned at the distal end of the endotracheal tube.1 Humidification of the ventilator circuit at physiologic temperature is important, particularly in long-term mechanical ventilation. To avoid rainout and circuit occlusion, modern ventilators use heated-wire humidification in the ventilator circuitry to warm all surfaces from the humidifier chamber to the patient Y-piece. Nebulizers can be inserted between the ventilator and the humidifier inlet (dry side) or before or after the heated-wire inspiratory limb of the circuit (wet side) and left in situ for repeated aerosol therapy. Nebulizer positioning has been well studied;2-4 it is known that the ventilator duty cycle can predict the ratio of inspired aerosolized material to expiratory phase losses when a jet nebulizer is used in the common placement 30.5 cm (12 inches) proximal to the Y-piece.1
Recent work by our group demonstrated that breath-enhanced jet nebulization from the wet, or outlet, side of the humidifier during mechanical ventilation provided predictable and reliable drug delivery.5-7 However, our subsequent long-term observations over 24 to 48 h demonstrated that both breath-enhanced jet and vibrating mesh nebulizers, when placed on the wet side of the humidifier and left in the circuit as single-patient use devices, become filled with condensed humidifier water vapor, which renders them unable to function.8 The Aerogen Solo nebulizer (Aerogen, Galway, Ireland) is often described to function on the dry, or inlet, side of the humidifier2,5,9 and is recommended for placement in this position by the manufacturer’s instructional literature.10
The present study, which used radiolabeled saline solution as an inhaled drug surrogate, was designed to evaluate the performance of a breath-enhanced jet nebulizer (i-AIRE nebulizer, InspiRx, Somerset, New Jersey) compared with a vibrating mesh nebulizer when situated on the dryside of the humidifier during adult mechanical ventilation. The protocol was designed to measure minute-by-minute drug delivery during both continuous infusion nebulization and bolus therapy. Measurement of the expiratory phase losses in relation to the inhaled mass (IM) further informed nebulizer efficiency at this location within the ventilator circuit by comparing the measured inspiratory to expiratory ratio (I:E) (ie, the aerosol delivery ratio) to that predicted by the duty cycle. To simulate potential clinical situations, aerosol delivery was also measured when the ventilator settings and infusion pump flow were changed during experimental runs (“dynamic continuous infusion” studies). Drug losses in the circuit were measured by mass balance by using a radioisotope calibrator and gamma camera.
Quick Look
Current Knowledge
Nebulization from the wet side of a heated humidified circuit during mechanical ventilation provides reliable and predictable drug delivery but may be limited by condensation in modern heated-wire circuits. When the nebulizer is placed at the patient Y-piece, aerosol losses are large and can be predicted by the ventilator duty cycle.
What This Paper Contributes to Our Knowledge
During adult mechanical ventilation, in vitro aerosol delivery by using bolus and continuous infusion nebulization from the dry side of a heated humidifier was predictable, with reduced expiratory losses. Aerosol delivery was controlled by infusion flow and initial ventilator settings. After achieving steady-state drug delivery, further adjustments to ventilator settings did not impact drug delivery.
Methods
Experimental Setup
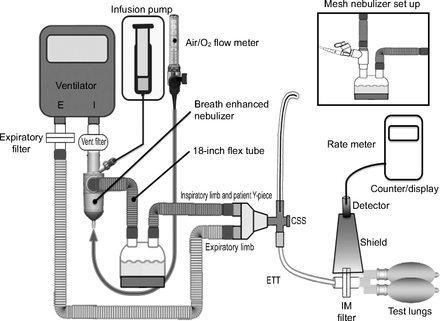
Measurement of real-time aerosol delivery during mechanical ventilation was recently developed in our laboratory to assess the effects of changing variables on drug delivery during continuous infusion nebulization,11 a treatment approach used in patients who are critically ill with severe hypoxemia or asthma. A typical experimental setup is outlined in Figure 1. The ventilator circuit was configured to replicate typical hospital circuits, whereby the patient Y-piece was attached to a Ballard closed-system suction device (Avanos Medical, Alpharetta, Georgia), a 7.5-mm inner diameter endotracheal tube (Rusch, Teleflex Medical, Morrisville, North Carolina) before connection to an IM collection filter (Pari, Starnberg, Germany) at the distal dip of the endotracheal tube to capture the aerosolized particles, which would be inhaled by a patient under similar conditions.
The ventilator circuit with the i-AIRE breath-enhanced jet nebulizer on the dry side of the humidifier. For the Solo nebulizer, the device was placed at the humidifier inlet port via the Aerogen T-piece (inset).
The ventilator was connected in parallel to a pair of 1-L neoprene test lungs via a Y-adapter immediately distal to the IM filter. The expiratory limb of the ventilator circuit was connected to an expiratory filter (Pari) just proximal to the ventilator’s exhalation port. The expiratory filter was used to collect and measure expiratory phase losses during aerosol delivery. The shielded ratemeter (Ludlum Measurements, Sweetwater, Texas) was positioned at the distal tip of the endotracheal tube at the level of the IM filter for real-time measurements of radiolabeled aerosol accumulating on the IM filter. Three different ventilators were used (Maquet Servo-i, Getinge, Sweden; Dräger, Telford, Pennsylvania; Avea CareFusion, Vyaire Medical, Mettawa, Illinois).
Tests were conducted by using the two adult breathing patterns listed in Table 1. These breathing patterns represent the extreme values of the range previously studied by our group during wet-side breath-enhanced jet nebulization.6 All the tests were performed using the volume-controlled continuous mandatory ventilation mode, with bias flow maintained at 2 L/min. Humidification was provided by a heated humidifier (MR-850 [Fisher & Paykel Healthcare, Auckland, New Zealand] or FL-9000U [Flexicare, Irvine, California]) and dual-limb heated-wire ventilation circuit (Fisher & Paykel Healthcare) to maintain fully saturated gas at 37 ± 1°C for all experiments, and was operated in the invasive ventilation mode with default settings. The temperature display was allowed to reach 37°C before the start of each experiment.
Ventilator Settings and Resultant Duty Cycles
Four InspiRx i-AIRE prototype breath-enhanced jet nebulizers and nine Aerogen Solo vibrating mesh nebulizers were used in rotation for all the experiments. Nine Aerogen Solo nebulizers were necessary to complete all the required experiments due to a 25% – 50% replacement rate. The i-AIRE nebulizer was installed in the ventilator circuit at the ventilator outlet port and connected to the inlet (dry side) of the humidifier by an 18-inch (45.7 cm) hose. The i-AIRE nebulizer was operated at 3.5 L/min by using compressed air at 50 psig. The Solo nebulizer was positioned in the ventilator circuit at the inlet to the humidifier via the Aerogen T-piece, connected according to the manufacturer’s instructions, and operated via the Aerogen Pro-X Controller. Solo nebulizers and the Pro-X controller were tested for proper performance before each testing sequence.
Technetium-99m pertechnetate radiolabeled normal saline solution was used for all of the experiments. For both types of continuous infusion experiments, a solution that contained 9 to 16 mCi of technetium-99m pertechnetate was drawn into a 60-mL syringe to achieve technetium-99m pertechnetate concentrations of 150 to 270 μCi/mL. For the bolus treatment experiments, 3- and 6-mL solutions that contained between 450 and 2,000 μCi were prepared for injection. Before the start of each experiment, the nebulizer was dry, empty, and free of radioactivity. For serial studies on the same day, 1 mL of non-radioactive normal saline solution was nebulized through the device after the completion of each experiment to remove any residual radioactivity from the previous test run.
Prior to each experiment, the radioactivity of the prepared solutions described above was measured with a radioisotope calibrator (Atom Lab 100, Biodex, Shirley, New York) to establish the exact initial charge. After aerosol delivery, the components of the circuit were measured by using a gamma camera (Maxi Camera 400, General Electric, Horsholm, Denmark; Power Computing, Model 604/150/D, Austin, Texas; Nuclear MAC OS, Version 4.2.2, Scientific Imaging, Thousand Oaks, California). The total volumes of the prepared solutions were precisely measured to determine the initial radioactivity per unit volume. The time at which this initial charge was measured served as the baseline time for decay correction of the subsequent measurements obtained throughout the experiment.
Drug Delivery Protocol
Our continuous infusion experiments were designed to test several approaches to therapy. Some studies that describe jet nebulizers initiated therapy with a prime by adding a specified quantity of drug solution to the nebulizer before the start of the infusion pump to provide immediate nebulization; other studies did not.12,13 Mesh devices do not require a prime. Furthermore, ventilator settings may need to be changed during a prolonged period of therapy. To assess these conditions, we designed 2 different protocols for testing the nebulizer function during continuous infusion. The first protocol, defined as “simple continuous infusion” prepared the i-AIRE nebulizer with a 2-mL prime of a radiolabeled saline solution at the start of infusion. This protocol established a data set for various starting conditions. For the second protocol, “dynamic continuous infusion,” which simulates real clinical scenarios, the i-AIRE nebulizer was not primed, and ventilator settings were deliberately changed during aerosol therapy as would occur during clinical treatment.
A programmable infusion syringe pump (BD Alaris Pump Module; Becton, Dickinson and Company, Franklin Lakes, New Jersey) was used to infuse radiolabeled saline solution into the nebulizer for a run time of 90 min for each simple continuous experiment. The i-AIRE nebulizer received a prime of 2 mL of radiolabeled saline solution at the start of infusion. The Solo nebulizer was not primed. Infusion pump flows of 5 and 10 mL/h were tested. In preliminary experiments that used a drop-by-drop infusion method, we found that the i-AIRE jet nebulizer required an infusion rate of at least 5 mL/h to provide steady-state aerosol delivery. In these preliminary experiments, it was observed that flows < 5 mL/h resulted in drying of the infusate and no nebulization; flows > 10 mL/h are near the maximum output.7 For this protocol, each infusion pump flow was tested with each of the 2 ventilator conditions (duty cycle 0.34 and duty cycle 0.13) as described above.
For dynamic continuous infusion experiments, the programmable infusion syringe pump was used as described above to infuse radiolabeled saline solution into the nebulizer for a run time of 220 min, which simulated prolonged patient therapy. The infusion rate was adjusted throughout each experiment based on a predetermined protocol that included predetermined ventilator setting adjustments. An ∼4-h protocol was chosen to model continuous infusion aerosol delivery in a more realistic scenario of a patient on mechanical ventilation.
Four separate sets of experiments were performed, each with a different starting condition (eg, a combination of pump flow and ventilator settings) identical to those used in the simple continuous infusion experiments described above. The predetermined protocol for pump flow and ventilator settings adjustments for each experimental set was chosen such that each set would experience a total of 5 distinct sequential conditions, including both ventilator and pump-flow changes, with return to the starting condition at the end of the set.
The bolus experiments were analogous to clinical delivery of bolus ampoules of drugs such as bronchodilators. The prepared 3- or 6-mL radiolabeled solutions were injected into the nebulizer before the start of the experiment. Of note, the fill volume for both nebulizers are similar, with a maximum operational volume of ∼6 mL. The end point of each experiment was determined by the cessation of nebulization detected by the ratemeter, which was visualized as a plateau in the real-time data collected.
Measurements
During each experiment, the shielded ratemeter positioned at the distal tip of the endotracheal tube was used to collect real-time measurements of the nebulized radioactivity delivered to the IM filter. The ratemeter was calibrated by using known sources of radioactivity measured separately in a radioisotope calibrator. The device was triggered manually and automatically captured 1-min counts of radioactivity, which were manually recorded into a spreadsheet to render a real-time graph of the IM filter counts against time.11 Data were collected at various time points beginning at 1 to 2 min after the start of the experiment through to the end of the experiment (90 min of continuous infusion, 220 min of dynamic continuous infusion, or completion of the bolus treatment). For the 90-min experiments, the total deposited radioactivity on the IM filter was also determined on the gamma camera, and the images were stored digitally for measurement and inspection. These experiments were performed in duplicate according to our protocol, except when the mesh nebulizer devices failed to empty, in which case, an additional run with a new device was added.
For the 220-min runs, the IM filter was changed at ∼90 min and 180 min to avoid effects of excess condensation on the filter. At the end of an experiment, the expiratory filter and nebulizer were measured to determine the expiratory phase aerosol loss and residual nebulizer radioactivity, respectively. Expiratory filter measurements were only reported for experiments with constant ventilator settings. IM data were calculated as a decay-corrected percentage of the original syringe activity (for infusion) or nebulizer charge (for bolus delivery) by using the known half-life of technetium and were reported as a function of time. For the longer dynamic infusion studies that required successive filters, these calculations were performed as described for each filter by using their own respective start and end times decay corrected back to time zero for the entire experiment. IM and expiratory data were reported as I:E.
In separate experiments, complete mass balance measurements were conducted for both the i-AIRE and Solo nebulizers to compare the total distribution of radioactivity deposited throughout the ventilator circuit. Clean, radioactivity-free circuit components were used for these experiments. At the end of the 90-min continuous nebulization run, each ventilator circuit component was separated and measured with the gamma camera. Mass balance percentages were calculated based on the amount of radioactivity delivered to the nebulizer during the test period. The images were digitally stored for measurement and inspection.
Analysis
Aerosol delivery, expressed as a percent of original nebulizer or infusion syringe charge as a function of time, was analyzed by using multiple linear regression. Nebulizer technology, the duty cycle, nebulizer residual radioactivity, and pump flow or bolus volume were assessed. All statistical analyses were performed by using GraphPad Prism 9.0 for Mac OS (GraphPad Software, San Diego, California). The influence of each variable was determined from the entire data set for each experimental condition (ie, simple continuous, dynamic continuous, and bolus) as follows: the R2 values for each independent variable were obtained by repeating the statistical analysis by using only one variable at first, then adding an additional variable in each iteration of analysis. In this way, the difference in R2 values from one iteration to the next accounts for the contribution of the additional variable that was included for that analysis and is reported as ΔR2 in the results tables. These values represent the percent contribution for each independent variable to the overall variability in drug delivery.14 The value of this analysis is seen by the magnitude of the regression coefficients, which define how much of the variability in output is ascribed to each independent parameter. Expiratory phase losses were analyzed as I:E and compared with the ratio predicted by the duty cycle.
Results
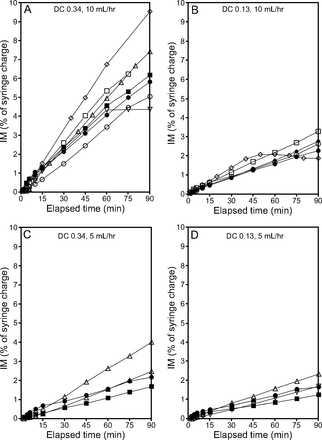
Data for all simple continuous infusion test runs are expressed as IM (percent of syringe charge) as a function of time. The infusion data for the i-AIRE and Solo nebulizers at 2 ventilator duty cycles (0.13 and 0.34) and with 2 different infusion pump flows (5 and 10 mL/h) are shown in Figure 2. IM increases linearly as a function of time for both devices. Visually, nebulizer curves overlap for both devices. The final IM at the end of the 90-min run increased with increasing pump flow, with more scatter at a higher duty cycle. The IM data for 25% of the Solo nebulizer experiments unexpectedly plateaued when the nebulizers stopped spontaneously and started to fill. This is consistent with previously published data.7 The data from these experimental runs were included in the analysis, and the devices subsequently were removed from circulation, were not used for future experiments, and were replaced with new nebulizers, which were pretested as per the manufacturer’s instructions.
The inhaled mass (IM) as a function of elapsed time for simple continuous infusion nebulization for the i-AIRE nebulizer (filled symbols) and Solo nebulizer (open symbols) at different duty cycle (DC) and pump flows. A: DC 0.34, flow 10 mL/h, B: DC 0.13, flow 10 mL/h, C: DC 0.34, flow 5 mL/h, and D: DC 0.13, flow 5 mL/h. All the data were plotted on the same scale; plateaus indicate a nebulizer that stopped nebulizing. Separate experiments are distinguished by using different symbols. The i-AIRE nebulizers were primed, and there is no separation between the i-AIRE and Solo nebulizer curves.
The average aerosol delivery and SD for both nebulizer types for each experimental condition are summarized in Table 2. Multiple linear regression analysis explained most of the variability in aerosol delivery (R2 = 0.793). Parameters that determined aerosol delivery were duty cycle (P < .001) and infusion pump flow (P < .001), with these two parameters accounting for 32% and 34% of the variability, respectively. The analysis revealed no significant effect of nebulizer type (P = .38) or the residual radioactivity remaining (P = .14) in the nebulizer at the end of each run. These data are shown in Table 3. Aerosol losses captured on the expiratory filter and the I:E for both nebulizers during simple continuous infusion are reported in Table 2. The average expiratory losses for the 4 sets of experimental conditions ranged from 0.9% to 1.0% and 1.2% to 2.5% of original syringe charge for the i-AIRE and Solo nebulizers, respectively. The average I:E for the 0.34 duty cycle experiments were 3.1 and 4.2 for the i-AIRE and Solo nebulizers, respectively; these ratios were several multiples higher than the ratio of 0.52 that would be predicted by a duty cycle of 0.34. Similarly, the average I:E for the 0.13 duty cycle experiments were 1.6 and 2.5 for the i-AIRE and Solo nebulizers, respectively. Again, these ratios were markedly higher than the ratio of 0.15 that would be predicted by the 0.13 duty cycle.
Average IM and Expiratory Mass Delivered in 90 min for Each Experimental Condition During Simple Continuous Infusion
Multiple Linear Regression With Inhaled Mass at 90 min (dependent variable) as a Function of Nebulizer Type, Infusion Pump Flow, Duty Cycle, and Nebulizer Residual for Simple Continuous Infusion, R2 = 0.793
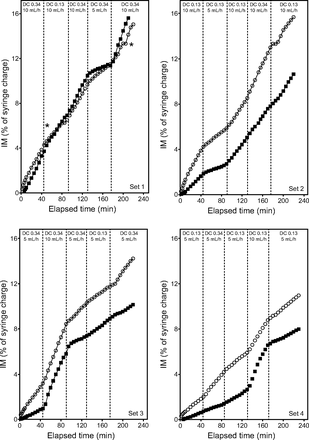
The data for all the dynamic continuous infusion test runs are expressed as IM (percent of syringe charge) as a function of time to show the amount of radiolabeled aerosol collected on the IM filter in real time during treatment, as shown in Figure 3. For each experiment, a predetermined series of duty cycle and pump flow changes were performed such that a change in aerosol delivered per minute can be visualized as a change in slope in Figure 3. Analysis of the data indicates linear aerosol delivery over time, with a steeper slope associated with initial settings of high pump flow and a long duty cycle. It is interesting to note that, once the initial ventilator and pump settings were set, the rate of aerosol delivery was only substantially affected by subsequent adjustments in the pump flow but not the ventilator settings. For example, the top left graph in Figure 3 shows initial conditions set as duty cycle 0.34 and pump flow of 10 mL/h, and the initial slope of the graph is, as expected, comparable with the slopes seen in the simple continuous infusion data for the same conditions (Fig. 2). After the system reached steady state at these initial conditions, the ventilator duty cycle was reduced from 0.34 to 0.13 by changing the ventilator settings, but there was no obvious change in the rate of aerosol delivery as visualized by the slope of the curve.
The inhaled mass (IM) as a function of elapsed time for dynamic continuous infusion for the i-AIRE (closed squares) and the Solo (open circles) nebulizers. Asterisks (*) indicate events in which the Solo nebulizer began to fill with liquid and required tapping to restart. The curves are separated due to an initial lag in i-AIRE nebulizer filling because these experiments did not use a nebulizer prime volume.
Similarly, the following ventilator adjustment back to duty cycle 0.34 did not seem to alter the rate of aerosol delivery. The proceeding adjustment of pump flow from 10 to 5 mL/h was associated with an immediate change in aerosol delivery, which is readily visualized by the change in slope. Finally, the system was returned to the initial conditions by increasing the pump flow to 10 mL/h, and there was a resultant increase in the slope of the curve, which was comparable with the slope at the start of the experiment. For the results shown in Figure 3, with lower pump flow and/or a short duty cycle, the i-AIRE nebulizer output was delayed because sufficient saline solution was needed to begin steady nebulization.11 This caused a separation in the output graphs between the Solo and i-AIRE nebulizers for three of the four test conditions in Figure 3 compared with Figure 2 (eg, i-AIRE nebulizers for the Fig. 2 experiments were primed). The slopes for all the sets of dynamic infusion experiments, which represent the rate of aerosol delivery, are summarized in Table 4.
The Rate of Aerosol Delivery (percent of syringe charge delivered per minute) for Each Set of Dynamic Continuous Infusion Experiments (Sets 1 – 4) with Predetermined Changes (A–E) in Conditions Listed in Sequential Order
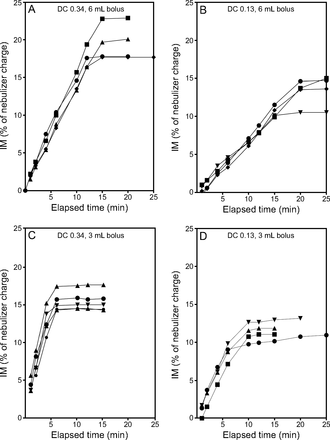
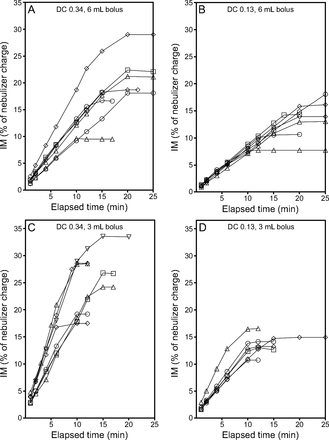
Multiple linear regression analysis of the rate of aerosol delivery indicated that 73% of the variability was accounted for by these tests (R2 = 0.732) (Table 5). Variability in the rate of aerosol delivery was independent of nebulizer type (P = .054) but dependent on pump flow (P < .001) and duty cycle (P = .003), with the latter two parameters accounting for 63% and 8% of the variability, respectively. Data for all bolus treatment test runs are expressed as IM (percent of syringe charge) as a function of time to show the amount of radiolabeled aerosol collected on the IM filter/min during treatment. Analysis of the data indicated linear aerosol delivery until the nebulizers emptied, at which point the data plateaus. Run times, as illustrated in Figures 4 and 5, varied from 5 to 20 min (i-AIRE nebulizer) and 5 to 25 min (Solo nebulizer), respectively. The average aerosol delivery and SD for both nebulizer types for each experimental condition are summarized in Table 6.
The inhaled mass (IM) as a function of elapsed time for bolus treatment nebulization by using the i-AIRE breath-enhanced jet nebulizer. Separate experiments are distinguished by using different symbols. A: Duty cycle (DC) 0.34, 6 mL bolus, B: DC 0.13, 6 mL bolus, C: DC 0.34, 3 mL bolus, and D: DC 0.13, 3 mL bolus.
The inhaled mass (IM) as a function of elapsed time for bolus treatment nebulization by using the Solo vibrating mesh nebulizer. Separate experiments are distinguished by using different symbols. The variation in the slopes and final outputs was primarily due to variation in the nebulizer residual because not all the devices emptied fully. A: Duty cycle (DC) 0.34, 6 mL bolus, B: DC 0.13, 6 mL bolus, C: DC 0.34, 3 mL bolus, and D: DC 0.13, 3 mL bolus.
Multiple Linear Regression with Inhaled Mass per Unit Time (dependent variable) as a Function of Nebulizer Type, Infusion Pump Flow, and Duty Cycle During Continuous Infusion With Dynamic Changes, R2 = 0.732
Average IM, Expiratory Mass, and I:E for Each Experimental Condition During Bolus Treatment
Multiple linear regression analysis (Table 7) indicated that 67% of the variability in delivery was accounted for (R2 = 0.672). Variability in aerosol delivery was independent of nebulizer type (P = .32) or bolus volume (P = .81). Variability in delivery was found to be dependent on the duty cycle (P < .001) and the residual radioactivity that remained in the nebulizer at the end of each run (P < .001), with these two parameters accounting for 39% and 20% of the variability, respectively. A second multiple linear regression analysis was performed with the nebulizer residual differentiated by nebulizer type, shown in Table 8. Again, the aerosol delivery was shown to be independent of the nebulizer type and bolus volume but dependent on the duty cycle (P < .001). Variability of the IM for both i-AIRE and Solo nebulizers was found to be a function of the residual activity, with the i-AIRE nebulizer residual contributing to 5% of the variability, and Solo nebulizer residual contributing 16%.
Multiple Linear Regression With Inhaled Mass (dependent variable) as a Function of Nebulizer Type, Bolus Volume, Duty Cycle, and Nebulizer Residual for Bolus Treatment, R2 = 0.672
Multiple Linear Regression With Inhaled Mass (dependent variable) as a Function of Nebulizer Type, Bolus Volume, Duty Cycle, and Nebulizer Residual Separated by Nebulizer Type for Bolus Treatment, R2 = 0.686
Aerosol losses captured on the expiratory filter and the I:E for the bolus delivery experiments are reported in Table 6. The average expiratory losses for the four sets of experimental conditions ranged from 6.5% to 0.8% and 6.2% to 8.9% of original syringe charge for the i-AIRE and Solo nebulizers, respectively. Average I:E for the 0.34 duty cycle experiments were 2.6 and 4.1 for the i-AIRE and the Solo nebulizers, respectively, and for the 0.13 duty cycle experiments, 2.0 and 3.0 for the i-AIRE and the Solo nebulizers, respectively. Again, these ratios are similar to those found in Table 2 and markedly higher than predicted by the corresponding duty cycles.
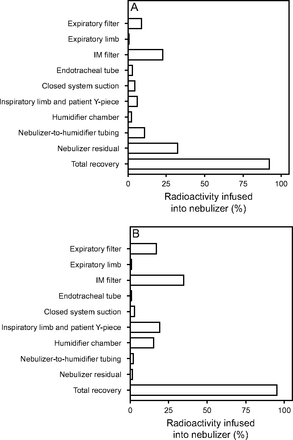
Mass balance experiments were performed on bothi-AIRE and Solo nebulizers by using the duty cycle 0.34 breathing pattern and a continuous infusion rate of 10 mL/h. The amount of aerosol recovered in each component of the ventilator circuit is shown Figure 6. The total recovery of aerosol was 92.5% for the i-AIRE nebulizer, and 95.4% for the Solo nebulizer. The results are reported as percentages of the amount of radioactivity infused by the syringe pump into the nebulizer over the 90-min period. Overall aerosol losses were similar for both nebulizers but differed in circuit distribution; 32.6% nebulizer residual for the i-AIRE nebulizer, with 11% in the tubing between the nebulizer and humidifier chamber. For the Solo nebulizer, 34.5% of the losses were attributed to the circuitry (19.3% in the inspiratory limb and patient Y-piece, 15.2% in the humidifier chamber), and 17% on the expiratory filter.
Mass balance data for the i-AIRE nebulizer (A) and the Solo nebulizer (B) Aerosol recovered in each ventilator circuit component is shown, with the total recovery at the bottom of each graph.
Discussion
The current bench study showed that dry-side nebulization is predictable in a modern heated-wire dual-limb ventilator circuit for both nebulizer technologies. The i-AIRE breath-enhanced jet nebulizer performed similarly to the Solo vibrating mesh nebulizer during both continuous infusion and bolus therapy. These observations suggest that the two different nebulizer technologies can be used interchangeably within existing hospital protocols for both intermittent treatments and continuous infusion aerosol delivery.
In previous reports, the i-AIRE nebulizer was more efficient on the wet side of the humidifier, with reduced residual volume, which suggests exposure to a humidified atmosphere increases nebulization.6 However, subsequent long-term observation demonstrated that unheated devices in fluid communication with humidified respiratory gases will eventually fill and trigger an occlusion alarm.8 Therefore, for single-patient use, wet-side nebulizer placement seems unacceptable for all single-patient use devices left in a circuit with a break in the heated-wire path. Placing single-patient use devices on the dry side eliminates this problem. Although Aerogen devices have been previously studied on the dry side by our group and others,2,11 we were not able to find a reason to avoid the wet side until we tested the configuration for days by exposing devices to continuous humidified ventilation.
There are further advantages of dry-side nebulization. Previous studies of nebulizer positioning showed that placement close to the patient Y-piece during continuous nebulization resulted in substantial aerosol losses to the expiratory limb directly from the inspiratory limb, bypassing the test lungs during all phases of the respiratory cycle except inspiration, with the duty cycle the major predictor of delivery.2,4,15 Placement distal to the Y-piece resulted in even less drug delivery.2 There also is concern for potential contamination from patient secretions with nebulizers placed near the endotracheal tube. Although there are some aerosol losses in the humidifier chamber (Fig. 6) when the nebulizer is placed on the dry side, both the humidifier and inspiratory limb tubing act as a reservoir for aerosol, which can be delivered to the patient during subsequent breaths.
Indeed, although it is known that the duty cycle predicts losses (a shorter duty cycle results in more deposition at the expiratory filter, as shown by the I:E),1,4 this effect was reduced in the present study, which supports the concept that the circuitry provides a reservoir. Specifically, for a nebulizer located distal to the Y-piece, a duty cycle of 0.34 predicts that 66% should be lost to the expiratory filter, or an I:E of 0.52, regardless of the infusion rate. Similarly, a duty cycle of 0.13 predicts that 87% should be lost to the expiratory filter, or I:E of 0.15. Lesser expiratory mass losses were found in the present study for both devices, as shown in Table 2 (simple continuous infusion, in which I:E ranged from 1.6 to 4.2 [predicted, 0.15 to 0.52]) and in Table 6 (bolus infusion, I:E ranged from 2.0 to 4.1 [predicted 0.15 to 0.52]). Again, analysis of these data supports the concept of the ventilator tubing and humidifier chamber as reservoirs for aerosols.
Circuit losses were also important, as demonstrated in the mass balance (Fig. 6). Although the Solo nebulizer results in more losses versus the i-AIRE nebulizer into the humidifier chamber (15.2% vs 2.3%), and inspiratory tubing (19.3% vs 6.2%), the losses are compensated by the overall higher efficiency of the Solo nebulizer when compared with the i-AIRE nebulizer, as illustrated by the differences in the nebulizer residual. Particle deposition throughout the circuit is likely affected by differences in aerosol particle size distributions and local flow effects. In recent granulometric studies we reported similar aerosol particle size distributions when measured at the distal endotracheal tube location,5,6 which suggests that the larger particles are filtered out of the aerosol by depositing in various circuit locations before the aerosol reaches the patient.
Two protocols for continuous infusion were studied. Some nebulizers in the literature are recommended to be primed.12,13 In the simple continuous infusion studies, the i-AIRE jet nebulizer was primed with 2 mL of radiolabeled saline solution before the start of the experiment, which allowed for rapid achievement of steady-state aerosol delivery. However, in a separate study by our group, it was observed that priming the i-AIRE jet nebulizer may lead to a transient high-dose delivery of aerosol to the IM filter.11 It was shown that, without priming, steady state was achieved within 5 – 20 min and would avoid the possibility of transient high dosing of a patient before attaining steady state. As such, a separate protocol without prime was adopted for the dynamic continuous infusion studies, which were designed to simulate a bedside situation in which priming of the i-AIRE nebulizer would not recommended. During simple continuous infusion, the variables that control drug delivery are the ventilator duty cycle (P < .001, R2 = 0.345) and infusion pump flow (P < .001, R2 = 0.321). The nebulizer technology and residual radioactivity remaining at the end of each run were not significant contributors (P = .38, ΔR2 = 0.070; and P = .14, ΔR2 = 0.056, respectively), which indicated that, despite the variability of the vibrating mesh nebulizer, the overall delivery of aerosol during continuous infusion did not show a statistically significant difference.
Dynamic continuous infusion studies were performed to mimic real clinical scenarios in which a patient may require ventilator adjustments during continuous long-term aerosol administration. Interestingly, it was observed in these dynamic studies that, once the starting condition had been set and steady-state aerosol delivery established, alterations in ventilator duty cycle did not result in a substantial change in the rate of aerosol delivery. This is similar to the results by Anderson et al,9 whereby there was no significant difference in deposition of continuously nebulized epoprostenol among different ventilator settings. The set of starting conditions of the dynamic studies were identical to the set of simple continuous infusion studies, and the rates of aerosol delivery were comparable (ie, the rate of delivery during the first set of conditions in any given dynamic infusion study is similar to the corresponding simple infusion study) and can be seen in Figures 2 and 3. Changes in the pump flow resulted in nearly immediate observable changes in delivery. These observations are noted for both the i-AIRE and Solo nebulizers.
Multiple linear regression analysis was consistent with the described observations; the duty cycle was only minimally contributory in the dynamic continuous infusion studies (P = .003, ΔR2 = 0.078), whereas the pump flow contributed 63% of the variability in aerosol delivery (P < .001, ΔR2 = 0.627). Again, the nebulizer type did not contribute significantly (P = .054, ΔR2 = 0.023). Although the rate of aerosol delivery can be accurately predicted based on set conditions, it is unclear at this time why the initial duty cycle impacts the rate of drug delivery, whereas subsequent changes render minimal effect. This will need further examination in future studies.
Analysis of our data demonstrated that drug dosing calculations based on body weight and infusion pump flow (such as those made by programmable infusion pumps) do not predict the actual dose delivered to the patient but serve as a starting point in mixing solutions. Our in vitro data can be viewed as a first approximation of dose delivered to the patient. First, device limitations define the range of pump flows. Preliminary observations of the i-AIRE by our group revealed that, at infusion flows below 5 mL/h, droplet evaporation exceeded droplet aggregation to the minimum volume necessary for nebulization. Priming the i-AIRE nebulizer resulted in a rapid attaining of a steady state within 5 min but could result in a transient high dose of aerosol delivery during this time. This observation would likely apply to any jet nebulizer; in contrast, without a prime, a steady state is reached in 5 to 20 min (steady state is reached faster with a higher pump flow and duty cycle) without the risk of a transient high dose delivered to the patient.11 For aerosol delivery to be sustained over time, the pump flow should be at least 5 mL/h. In addition, using a pump infusion rate beyond 10 mL/h is near to the limit defined by the i-AIRE nebulizer and 10 mL/h is similar to the maximum flow (12 mL/h) recommended for the Solo nebulizer by the manufacturer.10
These factors led to our recommendation that the infusion pump operate between 5 and 10 mL/h for the i-AIRE nebulizer. Once steady state is established, analysis of our data suggests that the ventilator can be adjusted as needed without concern for major deviation in the already established drug delivery. For inhaled medications, for example, epoprostenol, which are dosed based on ideal body weight, it is important to consider the influence of ventilator settings and device type on the actual administered dose.9,12,16,17 In a review by Dzierba et al,12 it was reported that the ideal operating dose range for aerosolized epoprostenol is 10 to 50 ng/kg/h based on ideal body weight. In such instances, appropriate adjustments in the delivered dose to the patient who is on ventilation should be made based on observed clinical response. In addition, nebulizer function should be periodically monitored. Failure to attain a clinical response may be associated with a device malfunction. We deliberately stopped our experiments at 90 or 220 min and reported residuals measured at that time. For those devices that plateau in output (ie, stop nebulizing), the nebulizer will eventually fill and should be replaced.
For bolus delivery (eg, for typical bronchodilator therapy), we found that drug delivery is independent of nebulizer type (P = .32, ΔR2 = 0.070) and bolus volume (P = .82, ΔR2 = 0.011). The variables that control aerosol delivery are the duty cycle (P < .001, ΔR2 = 0.394) and nebulizer efficiency, as represented by the residual radioactivity that remains at the end of the run (P < .001, ΔR2 = 0.197). These values are shown in Table 7. Due to visible differences noted in the residual between the 2 nebulizer types, statistical analysis was repeated on the same set of data with the residual separated by nebulizer type, shown in Table 8. The i-AIRE nebulizer residual, although higher in magnitude than that of the mesh nebulizer, exhibited less variability in drug delivery (P = .04, ΔR2 = 0.046) than the Solo nebulizer residual (P < .001, ΔR2 = 0.165). The variability in aerosol output for the mesh nebulizer device was primarily due to variation in the ability of individual devices to fully empty, as described in the statistical analysis in Table 8.
Inspection of Figures 2 and 5 reveals other differences between these technologies. The Solo nebulizer, if nebulization were complete in every run would deliver a higher average dose. In addition, for those mesh experiments in which nebulization was complete, one device may have a considerably different output than another, which implies intrinsic differences in mesh performance. This observation has been seen for other mesh nebulizers.18 The variability in the Solo nebulizer residual is reported in volumetric experiments elsewhere.19
A potential limitation of these studies was that the observation period of 220 min may not describe long-term continuous nebulization on the order of days. Although our observations did reach a steady state in a few minutes, device function over time in the hospital can run into many hours of therapy, which exceeded our period of observation. The findings described in this in vitro bench study should be confirmed clinically in future studies. There are other nebulizers available that are marketed for continuous nebulization, which were not studied. Other nebulizers marked for continuous infusion include the miniHEART (Westmed, Tucson, Arizona). The Solo nebulizer is the most heavily marketed in the United States and, therefore, was chosen as the comparison device for this study. Future studies could also include simple jet nebulizers without breath enhancement as a comparator. Only two ventilator duty cycles, chosen as the extremes of usual operation settings, were studied. Other ventilator parameters that may contribute to expiratory losses and delivery of the IM were not studied, including bias flow. A potential limitation of the i-AIRE device was the narrow range of pump flow (5 – 10 mL/h) that can be supported within the studied ventilator settings.
Conclusions
Nebulizer technology combined with changes in operation of humidifiers and ventilator circuits have resulted in important changes in aerosol delivery. This study offers a planned approach to therapy that allows the therapist to affect treatment beyond just filling the nebulizer. Ventilator factors and infusion pump flows can affect drug delivery and may have predictable effects on the clinical response.
Footnotes
- Correspondence: Janice A Lee MD, HSC T17-040, Division of Pulmonary, Critical Care and Sleep Medicine, Stony Brook School of Medicine, 101 Nicolls Road, Stony Brook, NY 11794–8172. E-mail: Janice.lee{at}stonybrookmedicine.edu
See the Related Editorial on Page 1064
The study was supported, in part, by InspiRx, Somerset, New Jersey. The State University of New York at Stony Brook holds patents in the fields of nebulizer development and inhaled antibiotic delivery that have been licensed to InspiRx. Dr Smaldone and Ms Cuccia disclose relationships with InspiRx. Dr Lee and Mr McPeck have disclosed no conflicts of interest.
A version of this paper was presented in part by Dr Janice A Lee at the International Society for Aerosols in Medicine Congress, held May 22-26, 2021, in Boise, Idaho.
- Copyright © 2022 by Daedalus Enterprises