Abstract
An animal suspension model has been used to simulate the weightlessness experienced during space travel. This procedure results in a reduction in the normal shortening (i.e. hypokinesia) and force generation functions of hind limb muscles (i.e. hypodynamia). The ensuing muscle atrophy was studied over 12 days in different muscle types. Slow muscles (e.g. the soleus) underwent a more pronounced atrophy than intermediate (i.e. gastrocnemius) and fast phasic muscles (e.g. extensor digitorum longus). In all muscle types inactivity resulted in a smaller accumulation of DNA and losses of RNA and protein after 5 days. The latter arose from a decrease in the rate of protein synthesis (measured in vivo) and an increase in protein breakdown. Increased specific activities of cathepsins B and D also supported the view that there is an increased proteolysis after hypokinesia and hypodynamia.
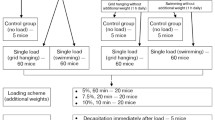
When the inactive soleus was simultaneously held in a lengthened (stretched) state the atrophy was prevented through a large increase in the fractional rate of protein synthesis. Protein degradation remained elevated with stretch, thereby slowing the growth of these muscles relative to those in pair-fed, ambulatory controls. The much smaller atrophy of the tibialis anterior and extensor digitorum longus muscles in suspended only limbs represented an underestimate of the true atrophic effects of hypokinesia and hypodynamia. In this model gravity pulls the suspended foot into a plantar flexed position, thereby permanently stretching and protecting such flexor muscles. When this influence of stretch was removed a greater atrophy ensued, mainly due to the loss of the stretch-induced stimulation of protein synthesis. Despite this, the inactive fast-twitch muscles still exhibited less atrophy than the gastrocnemius and soleus muscles.
Similar content being viewed by others
References
Ariano MA, Armstrong RB, Edgerton VR (1973) Hindlimb muscle fibre populations of five mammals. J Histochem Cytochem 21:51–55
Barrett AJ (1980) Fluorimetric assays for cathepsin B and cathepsin H with methylcoumarylamide substrates. Biochem J 187:909–912
Booth FW, Nicholson WF, Watson PA (1982) Exercise and sports science reviews, vol 10. Franklin Institute Press, Philadelphia, pp 27–48
Chiu LA, Castleman KR (1980) Morphometric analysis of rat muscle fibres following space flight and hypogravity. Physiologist 23:S76-S78
Feller DD, Ginoza HS, Morey ER (1981) Atrophy of rat skeletal muscles in simulated weightlessness. Physiologist 24:S9-S10
Garlick PJ, Fern EB, McNurlan MA (1979) Methods for determining protein turnover. In: Rapoport S, Schewe T (eds) Processing and turnover of proteins and organelles in the cell, vol 53. Proc. FEBS. Pergamon Press, Oxford-New York, pp 85–94
Garlick PJ, McNurlan MA, Preedy VR (1980) A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of (H)3 phenylalanine. Biochem J 192:719–723
Goldberg AL, Goldspink, DF (1975) Influence of food deprivation and adrenal steroids on DNA synthesis in various mammalian tissues. Am J Physiol 228:310–317
Goldspink DF (1977) The influence of immobilization and stretch and protein turnover of rat skeletal muscle. J Physiol 264:267–282
Goldspink DF (1978) The influence of passive stretch on the growth and protein turnover of the denervated extensor digitorum longus muscle. Biochem J 174:595–602
Goldspink DF (1980) Physiological factors influencing protein turnover and muscle growth in mammals. In: Goldspink DF (ed) The development and specialization of skeletal muscle. Cambridge University Press, pp 65–89
Goldspink DF, Lewis SEM (1985) Developmental changes and the influence of exercise, stretch and innervation on muscle growth. In: Glaumann H, Ballard FJ (eds) Lysosomes: Their role in protein breakdown. Academic Press (in press)
Goldspink DF, Lewis SEM, Kelly FJ (1985) Protein turnover and cathepsin B activity in several individual tissues of foetal and senescent rats. Comp Biochem Physiol 82 B, 849–853
Goldspink G, Tabary JC, Tabary C, Tardieu G (1974) Effect of denervation on the adaptation of sarcomere number and muscle extensibility to the functional length of the muscle. J Physiol 236:733–742
Holly RG, Barnett JG, Ashmore CR, Taylor RG, Mole PA (1980) Stretch-induced growth in chicken wing muscles: A new model of stretch hypertrophy. Am J Physiol 238:C62-C71
Kazaryan VA, Rapoport EA, Goncharova LA, Bulychewa SY (1977) Effect of prolonged weightlessness on protein metabolism in red and white skeletal muscles of rats. Kosm Biol Aviakosm Med 11:19–23
Loughna PT, Goldspink G, Goldspink DF (1986a) Protein turnover in phasic and postural hindlimb muscles of the rat in response to hypokinesia, hypodynamia and passive stretch. Am J Appl Physiol (in press)
Loughna PT, Cook P, Goldspink DF, Goldspink G (1986b) Muscle fibre atrophy during disuse and its prevention by passive stretch. Cell Tissue Res (in press)
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Morey ER (1979) Spaceflight and bone-turnover-correlation with a new rat model of weightlessness. Bioscience 29:168–172
Morey ER, Baylink DJ (1978) Inhibition of bone formation during space flight. Science 201:1138–1141
Morey-Holton E, Wronski WJ (1981) Animal models for simulating weightlessness. Physiologist 24:S45-S48
Musacchia XJ, Steffen JM, Deavers DR (1981) Suspension restraint: reduced hypokinesia antiorthostasis as a simulation of weightlessness. Physiologist 24:S21-S22
Musacchia XJ, Deavers DR, Meininger G, Davis TP (1980) A model for hypokinesia: effects on muscle atrophy in the rat. J Appl Physiol 48:479–486
Palmer RM, Reeds PJ, Atkinson T, Smith RH (1983) The influence of changes in tension on protein synthesis and prostaglandin release in isolated rabbit muscles. Biochem J 214:1011–1014
Pennington RJT (1977) Proteinases of muscle. In: Barrett AJ (ed) Proteinases in mammalian cells and tissues. North Holland, Amsterdam, pp 515–543
Pette D (ed) Plasticity of muscle. Walter de Gruyter, Berlin New York
Pluskal MG, Pennington RJ (1973) Peptide hydrolase activities in denervated muscle. Biochem Soc Trans 1:1307–1310
Rodemann HP, Goldberg AL (1982) Arachidonic acid, prostaglandin E2 and F2 influence rates of protein turnover in sceletal and cardiac muscle. J Biol Chem 257:1632–1638
Sargeant AJ, Davies LTM, Edwards RHT, Maunder C, Young A (1977) Functional and structural changes after disuse of human muscle. Clin Sci Mol Med 52:337–342
Shear CR (1978) Cross sectional myofibre and myofibril growth in immobilized developing skeletal muscle. J Cell Sci 29:297–312
Templeton GH, Padalino M, Manton J, Glasberg M, Silver CJ, Silver P, De Martino G, Leconey T, Klug G, Hagler H, Sutko JL (1984) Evaluation to the response of rat skeletal muscle to a model of weightlessness. Physiologist 25:S513-S514
Tischler ME, Jaspers SR (1982) Synthesis of amino acids in weight bearing and non weight bearing leg muscles of suspended rats. Physiologist 25:S155-S156
Waterlow JC, Garlick PJ, Millward DJ (eds) (1978) Protein turnover in mammalian tissues and in the whole body. North Holland, Amsterdam, pp 625–696
Watson PA, Stein JP, Booth FW (1984) Changes in actin synthesis and actin-mRNA content in rat muscle during immobilization. Am J Physiol 247:C39-C44
Williams PE, Goldspink G (1973) The effect of immobilization on the longitudinal growth of striated muscle. J Anat 116:45–55
Williams PE, Goldspink G (1978) Changes in sarcomere length and physiological properties in immobilized muscle. J Anat 127:459–468
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Goldspink, D.F., Morton, A.J., Loughna, P. et al. The effect of hypokinesia and hypodynamia on protein turnover and the growth of four skeletal muscles of the rat. Pflügers Arch. 407, 333–340 (1986). https://doi.org/10.1007/BF00585311
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00585311