Abstract
Objective
To compare the effects of He/O2 and external PEEP (PEEPe) on intrinsic PEEP (PEEPi), respiratory mechanics, gas exchange, and ventilation/perfusion (V̇A/Q̇) in mechanically ventilated COPD patients.
Design and setting
Prospective, interventional study in the intensive care unit of a university hospital.
Interventions
Ten intubated, sedated, paralyzed, mechanically ventilated COPD patients studied in the following conditions: (a) baseline settings made by clinician in charge, air/O2, ZEEP; (b) He/O2, ZEEP; (c) air/O2, ZEEP; (d) air/O2, PEEPe 80% of PEEPi. Measurements at each condition included V̇A/Q̇ by the multiple inert gas elimination technique (MIGET).
Results
PEEPi and trapped gas volume were comparably reduced by He/O2 (4.2±4 vs. 7.7±4 cmH2O and 98±82 vs. 217±124 ml, respectively) and PEEPe (4.4±1.3 vs. 7.8±3.6 cmH2O and 120±107 vs. 216±115 ml, respectively). He/O2 reduced inspiratory and expiratory respiratory system resistance (15.5±4.4 vs. 20.7±6.9 and 19±9 vs. 28.8±15 cmH2O l−1s−1, respectively) and plateau pressure (13±4 vs. 17±6 cmH2O). PEEPe increased airway pressures, including total PEEP, and elastance. PaO2/FIO2 was slightly reduced by He/O2 (225±83 vs. 245±82) without significant V̇A/Q̇ change.
Conclusions
He/O2 and PEEPe comparably reduced PEEPi and trapped gas volume. However, He/O2 decreased airway resistance and intrathoracic pressures, at a small cost in arterial oxygenation. He/O2 could offer an attractive option in COPD patients with PEEPi/dynamic hyperinflation.
Similar content being viewed by others
Introduction
In mechanically ventilated patients with chronic obstructive pulmonary disease (COPD), incomplete exhalation of inspired tidal volume (VT) due to elevated airway resistance and decreased lung elastic recoil can lead to an increase in end-expiratory lung volume, termed "dynamic hyperinflation" [1, 2] or "intrinsic" positive end-expiratory pressure (PEEPi) [3, 4]. The numerous deleterious effects of PEEPi on respiratory mechanics, gas exchange, hemodynamics, oxygen transport, and work of breathing [3, 4, 5] can be attenuated by measures aimed at reducing dynamic hyperinflation, such as reduction in VT and respiratory rate [6], bronchodilators [7], and applying external PEEP (PEEPe) [8]. However, the latter is difficult to titrate [4] and may by itself be detrimental by increasing lung volumes and intrathoracic pressures [9, 10], and worsening of hemodynamics [9, 11]. Alternatively, replacing the inhaled air-oxygen mixture by helium-oxygen, which reduces resistance to flow in the airways [12] due to its low density, has been shown to decrease PEEPi and trapped gas volume [13]. However, He/O2 also raises concerns, among which are interference with ventilator function [14] and worsening of hypoxemia, the latter having been documented in spontaneously breathing COPD patients [15, 16]. The purpose of this study was thus to compare the effects of He/O2 and PEEPe on PEEPi, respiratory mechanics, gas exchange, and ventilation/perfusion (V̇A/Q̇) in mechanically ventilated COPD patients.
Methods
Patients
The study was conducted in the Division of Intensive Care, St.-Luc Hospital, Brussels. Intubated patients consecutively admitted to the ICU were included if they met diagnostic criteria of COPD [17] and had been mechanically ventilated for no longer than 48 h. Patients were excluded if pneumothorax was present or the inspired O2 fraction (FIO2) was 0.4 or higher. The study included ten patients (aged 64±9 years) after a mean of 28±5 h of mechanical ventilation. Individual baseline characteristics, main ventilator settings, PEEPi measurement and arterial blood gases of the patients are summarized in Table 1. The study protocol was approved by the ethics committee of the Catholic University of Louvain, Brussels. Consent was obtained from next of kin.
Procedures and measurement techniques
Patients were sedated and paralyzed by a continuous infusion of propofol or midazolam, and vecuronium or atracurium. All patients were ventilated with a Siemens Servo 300 (Siemens-Elema, Solna, Sweden) which is easily compatible with the use of helium [14]. Helium was delivered from a 50-l canister containing a 78:22 mixture of He and O2, pressurized at 200 bar, through a pressure regulator at 6 bar into the ventilator's air inlet [18]. Ventilator mode was volume-controlled pressure limited ventilation. Set VT was not corrected for He/O2 since on the machine used the change in density does not affect delivered VT, while expired VT (VTe) readings were corrected using appropriate factors [14]. As a final precaution VTe was monitored with a density-independent spirometer (5420 Volume Monitor, Ohmeda, Louisville, Col., USA).
During the entire protocol, the FIO2 and ventilator settings made by the clinician in charge of the patient before inclusion were kept constant. Maximum pressure limit was set at 40 cmH2O. Inspiratory flow rate was 60 l/min, square wave flow pattern. No PEEP was set on the ventilator except during the last step of the study at which time an PEEPe of 80% of PEEPi measured at air/O2 zero end-expiratory pressure (ZEEP) 2 was applied (see below).
Respiratory rate, airway pressure, flow, and inspiratory:expiratory (I:E) ratio were recorded from the ventilator. Respiratory system static elastance (Ers) and inspiratory (Rinsp) and expiratory (Rexp) resistances were computed by the automatic measuring algorithms of the ventilator after a brief end-inspiratory pause. PEEPi was measured by an end-expiratory occlusion [3], also performed automatically on the Servo 300. This end-expiratory occlusion technique actually measures total PEEP in static conditions. Therefore when no PEEPe is applied, the readout is equivalent to the value of PEEPi, whereas when PEEPe is applied, PEEPi is equal to the value of total PEEP (PEEPtot) obtained by end-expiratory occlusion minus that of PEEPe set on the ventilator [4, 19]. For each set of measurements the end-expiratory occlusion was performed three times at 1-min intervals and PEEPi reported as the mean of the three readings. End-expiratory trapped lung volume (Vtrapped) was determined by the end-inspiratory apnea technique [6]. Briefly, the patient was disconnected from the ventilator at end-inspiration, and the total exhaled volume measured with the spirometer, until expiratory flow was no longer detectable. Total exhaled volume represents total end-inspiratory volume (VEI) above functional residual capacity. Vtrapped at end-expiration was then computed as: Vtrapped=VEI−measured VT. Measured VT was determined as the mean of the last five breaths before the maneuver [6].
Heart rate and mean systemic arterial pressure were continuously monitored by standard three-lead monitoring electrodes and an indwelling arterial catheter, respectively. Arterial oxygen saturation was continuously monitored by pulse oximetry.
Ventilation-perfusion relationships by the multiple inert gas elimination technique
The distribution of the V̇A/Q̇ values was assessed by the multiple inert gas elimination technique (MIGET) [20]. Briefly, six inert gases of varying solubility (SF6, ethane, cyclopropane, halothane, ether, and acetone) were equilibrated in 0.9% NaCl and were infused at a constant rate of 3 ml/min through the central venous line. After a 30-min equilibration period 10 ml blood samples from the peripheral artery and 5 ml blood samples from the pulmonary artery catheter, if present, or the central venous line were withdrawn into 20-ml heparinized glass syringes. Central venous sampling is an acceptable alternative in the absence of a pulmonary artery catheter [21]. Samples of mixed expired gas were collected from the exhaust port of the ventilator into 50 ml gas-tight syringes (Hamilton 50 TLL, Hamilton, Reno, Nev., USA). A gas chromatograph (Perkin Elmer, Shelton Conn., USA) equipped with an electron capture detector for SF6 and a flame ionization detector for the other five gases was used to determine the inert gas concentrations. Retention (ratio of arterial to mixed venous concentration) and excretion (ratio of mixed expired air to mixed venous concentration) were computed for each gas. The continuous distribution of blood flow and ventilation against the ventilation-perfusion ratios from these data were calculated by the computer program developed by Evans and Wagner [22]. Disp R-E*, an overall index of V̇A/Q̇ heterogeneity, was also determined. The residual sum of squares, a quantitative estimation of the overall experimental error in the procedure, was computed [23]. In high-quality measurements the residual sum of squares between the measured and calculated V̇A/Q̇ distributions should be less than 5.3 in 50% and less than 10.6 in 90% of all data sets [23].
Cardiac output and oxygen transport
If a pulmonary artery catheter was in place, cardiac output was determined by thermodilution. In the absence of such a catheter, cardiac output was estimated by the Fick method, using central venous rather than mixed venous blood samples. Oxygen transport (ḊO2) was computed according to standard equations. Oxygen consumption (V̇O2) was determined from the inspired-expired O2 concentrations.
Measurement protocol
A complete set of all measurements was performed at the following time points: (a) upon starting the protocol, no PEEPe (air/O2 ZEEP1); (b) after 30 min of He/O2 inhalation, no PEEPe (He/O2 ZEEP); (c) after 30 min of air/O2 inhalation, no PEEPe (air/O2 ZEEP 2); (d) after 30 min of air/O2 inhalation, PEEPe, set as 80% of PEEPi measured at air/O2 ZEEP 2 (air/O2 PEEPe). The mean level of PEEP applied at air/O2 PEEPe was 6.4±4 cmH2O.
Statistical methods
Values are reported as mean ±standard deviation. One-way analysis of variance for repeated measures was used to compare the values obtained at each of the four conditions. Significance between time points was determined by Fisher's protected least significance test. A p value less than 0.05 was considered significant.
Results
Ventilatory parameters PEEPi and respiratory mechanics
As shown in Table 2, He/O2 led to a significant reduction in peak (PIP) and plateau (Pplat) pressures, Ers, and both Rinsp and Rexp. Conversely, PEEPe significantly increased mean airway pressure and Pplat, while there was a trend towards a rise in PIP and Ers. Rinsp and Rexp were not significantly affected by PEEPe. Vtrapped and PEEPi were both comparably reduced by He/O2 and PEEPe (Table 2), this response being present in all patients, as shown in Fig. 1. However, PEEPtot differed markedly between these two conditions (Fig. 2). With He/O2, as expected, since PEEPi was reduced in all patients and no PEEPe was applied, PEEPtot was also decreased in all patients. With PEEPe, even though PEEPi decreased in all patients, PEEPtot decreased in only two (patients 5 and 7), was unchanged in three (patients 1, 6, and 9), and increased in five (patients 2, 3, 4, 8, and 10), as shown in Fig. 2.
Intrinsic PEEP and trapped gas volume. Individual values of trapped gas volume (Vtrapped, left) and intrinsic PEEP (PEEPi, right) under all conditions tested. Air/O 2 ZEEP 1 Initial settings made by clinician, no PEEP; He/O 2 ZEEP after 30 min of He/O2 inhalation, no PEEPe; air/O 2 ZEEP 2 after 30 min of air/O2 inhalation, no PEEP; air/O 2 PEEPe after 30 min of air/O2 inhalation, PEEPe 80% of PEEPi measured at air/O2 ZEEP 2
Total and external PEEP. Individual measured levels of total PEEP (PEEPtot), determined by the end-expiratory occlusion technique, during He/O2 inhalation (A, left) and external PEEP (PEEPe) application (B, right). A Magnitude of PEEPtot (i.e., intrinsic PEEP, since no PEEPe was applied) during air/O2 (white bars) and He/O2 (black bars) inhalation. B Magnitude of PEEPtot with air/O2 and ZEEP (white bars), and air/O2 and PEEPe application, partitioned into PEEPi (black area) and PEEPe (hatched area) components
Arterial blood gases and V̇A/Q̇ relationships
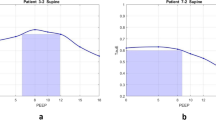
No significant modification in arterial pH or arterial blood gases was observed during the study, with the exception of a decrease in arterial oxygenation during He/O2 inhalation (Table 3). MIGET analysis (Table 4) showed a small shunt at baseline, with most of the perfusion directed to areas of intermediate or normal V̇A/Q̇. A high deadspace fraction was present at baseline, the remaining ventilation being distributed mainly to areas of intermediate or normal V̇A/Q̇. He/O2 exerted little change on V̇A/Q̇ relationships. No significant V̇A/Q̇ modification was observed with PEEPe. MIGET tracings from a typical patient are shown in Fig. 3. V̇A/Q̇ heterogeneity, quantified by the Disp R-E* index, was not significantly modified by either He/O2 or PEEPe (Table 4). Mean overall residual sum of squares was 3.02±1.84, and was less than 5.3 in 80% of data sets, well within the range of high technical quality measurements [23].
Hemodynamics and oxygen transport
No significant changes in arterial blood pressure, heart rate ḊO2, or V̇O2 were noted during the various phases of the study (Table 3)
Complications
No complication occurred during any of the protocol phases.
Discussion
The main findings of this study in a group of sedated, paralyzed, and mechanically ventilated COPD patients with moderate levels of PEEPi are that He/O2 reduced PEEPi and Vtrapped, airway pressures and resistances, and elastance at a small cost in arterial oxygenation. Conversely, PEEPe set at 80% of measured PEEPi, reduced PEEPi and Vtrapped but increased airway pressures and in most patients PEEPtot. Neither approach significantly affected V̇A/Q̇ distribution.
Effects of He/O2
The observed effects of He/O2 on PEEPi and respiratory mechanics are consistent with those documented in a previous study in intubated, sedated, paralyzed patients undergoing controlled mechanical ventilation, with comparable levels of PEEPi, in which He/O2 led to a marked decrease in Vtrapped and PEEPi in 22/23 patients [13]. The concordant results from the two studies, with effects observed in almost every patient, provides further evidence that He/O2, due to its low density and resultant reduction in airway resistance, effectively attenuates dynamic hyperinflation/PEEPi in this setting. It should be noted nonetheless that He/O2 has no impact on the cause of obstructive disease and airflow limitation. Thus its effects disappear once its administration is discontinued, as shown by the rapid return to baseline values of PEEPi and Vtrapped in both our studies when He/O2 inhalation was stopped. Consequently the use of He/O2 should not deter ICU physicians from aiming to decrease airway obstruction with bronchodilating drugs, and avoiding excessive respiratory rate and VT settings on the ventilator [4]. Among concerns regarding the use of He/O2 in COPD patients is a possible worsening of arterial oxygenation, documented in studies performed in spontaneously breathing nondecompensated patients [15, 16]. In one study He/O2 breathing entailed a decrease in PaO2, hypothesized to result from an increase in the heterogeneity of V̇A/Q̇ distribution [16]. Another study documented an increase in the alveoloarterial O2 gradient during He/O2 inhalation [15]. MIGET analysis was consistent with a diffusion impairment for O2, attributed by the authors to a proximal displacement of the transition from convective to diffusive gas transfer processes [15, 24]. Finally, a slight decrease in PaO2 with He/O2 compared to air/O2 was also noted in a study by Christopherson and Hlastala [25], in mechanically ventilated dogs, without significant change in MIGET results, and also attributed by the authors to displacement of the convective/diffusive front in the airways [15, 24, 25]. However, whether these results can be extrapolated to decompensated and mechanically ventilated patients is unclear since in a previous study on patients undergoing mechanical ventilation we found no impact of He/O2 on arterial oxygenation [13]. It should also be noted that a decrease in alveolar ventilation, suggested by the slight rise in PaCO2 with He/O2, could also have contributed in the decrease in PaO2.
Our MIGET results are in line with these observations. Overall the baseline pattern of a small fraction of shunt and perfusion to low V̇A/Q̇ regions, perfusion predominating in regions of V̇A/Q̇=1, and high deadspace (Table 4) is consistent with both the so-called "H" pattern described by Wagner et al. [26] in stable spontaneously breathing patients with severe COPD and the profile found in two studies in COPD patients during controlled mechanical ventilation [8, 27]. In our study no significant change was observed with He/O2, thus excluding a major effect of He/O2 on shunt, low V̇A/Q̇ or worsening of V̇A/Q̇ inequality. The convective/diffusion front theory mentioned above could explain these results [24], the small magnitude of worsening hypoxemia being in line with that observed in other studies [15, 25]. Indeed, the magnitude of worsening of hypoxemia was small (8%) and is probably of negligible clinical importance in patients receiving a mean FIO2 of 0.35. Why these findings differed from those of our preceding study [13] in a comparable patient population and setting is not immediately clear. However, in the earlier study patients were ventilated for 45 min with He/O2, compared to the 30 min in the present study, possibly allowing any time-dependent short-term V̇A/Q̇ heterogeneity to subside. Furthermore, there was a trend towards a decrease in PaO2 in the former study with He/O2, by 6%, although the difference was not statistically significant [13]. Of importance, and in the same line of thought, no worsening of hypoxemia was noted during noninvasive pressure support with He/O2 in decompensated COPD patients in two recent studies [18, 28]. Finally, a recent study on the impact of various inspiratory flow waveforms in mechanically ventilated COPD patients demonstrated that square wave inspiratory flow as used in the present study was less favorable on gas exchange than decelerating flow [29]. This factor might have contributed to our results. To summarize, it seems that a reduction in PaO2 during He/O2 inhalation in this setting is either absent or of very small magnitude and probably represents a minor price to pay for the major beneficial effects on dynamic hyperinflation and respiratory mechanics.
Regarding PaCO2, the absence of change with He/O2 was somewhat surprising, given that two studies using noninvasive ventilation documented a reduction in PaCO2 with He/O2, possibly due to improved CO2 diffusion [18, 28]. However, the results are in accord with those of our previous study in intubated and mechanically ventilated patients [13] and are in line with the absence of change in the V̇A/Q̇ results, in particular deadspace (Table 4).
Effects of PEEPe
In patients with PEEPi undergoing spontaneous/assisted mechanical ventilation, applying PEEPe has been shown to reduce the inspiratory threshold load, ease triggering of the ventilator, and reduce work of breathing [30, 31]. However, any benefit of PEEPe during controlled ventilation is much less obvious [4], as underlined in a recent publication [19] and as demonstrated in various studies [8, 11, 32]. In a study using the MIGET in COPD patients during controlled ventilation, Rossi et al. [8] showed that when PEEPe at 50% of measured PEEPi was applied, no change in respiratory mechanics was noted, while PaO2 increased as a result of a rise in the mean value of the distribution of perfusion. However, when PEEPe equivalent to 100% of PEEPi was applied, airway pressures rose, and no further improvement in gas exchange was noted [8]. It should also be mentioned that no change in oxygen transport was noted with the application pf PEEPe, while reducing PEEPi through controlled hypoventilation increased cardiac output and oxygen transport [8]. This could have resulted from a reduction of the adverse hemodynamic effects of PEEPi and could also be observed when PEEPi is decreased by He/O2. However, we made no such observation in our patients, probably because there appeared to be little hemodynamic impact from PEEPi, as observed in a prior study [13]. Baigorri et al. [11] showed that applying a PEEPe equal to measured PEEPi led to an increase in end-expiratory volume, a rise in intrathoracic pressures, and no improvement in arterial blood gases, while a decrease in cardiac output was noted with a PEEPe exceeding PEEPi. Fernandez et al. [32] observed that the increase in end-expiratory volume when setting PEEPe equal to PEEPi was directly proportional to respiratory system compliance, and hence that its magnitude was difficult to predict in routine clinical conditions. The reasons for this lie in the fact that in the presence of expiratory flow limitation added increments of PEEPe progressively replace PEEPi, without increasing total PEEP and lung volume, until a critical value of PEEPe is reached, above which total PEEP and lung volume both increase [33, 34]. When the latter occurs, increased respiratory system elastance, decreased cardiac output and worsening of gas exchange occur [34].
These various issues have led to the recommendation of either refraining from using PEEPe in the presence of PEEPi during controlled mechanical ventilation [19], or to not exceed values of 50–85% of measured PEEPi, while carefully monitoring the consequences of its application [4]. In our study the goal was to apply a PEEPe equivalent to 80% of PEEPi. However, it is difficult to ascertain that this goal was always attained, since although we used the PEEPi measured during the third step (air/O2 ZEEP 2), it is well known that PEEPi can change fairly quickly [35]. Thus it is possible that PEEPe levels equal to or exceeding PEEPi were applied in some patients, as our results suggest. Indeed, as shown in Fig. 2, in most patients PEEPi was replaced by PEEPe, but total PEEP was mainly unchanged or even increased. This observation is in line with the studies cited above demonstrating that, in the absence of expiratory flow limitation, or if excessive PEEPe levels are used even if such a limitation is present, worsening dynamic hyperinflation and its complications can occur. A seminal study by Tuxen [9] has illustrated how severe the latter can be. Interestingly, in the latter study, as overall lung volume and total PEEP increased when high levels of PEEPe were applied, Vtrapped decreased, most likely due to the rise in elastance and a decrease in airway resistance associated with the higher lung volume [9]. A similar observation was made in our patients (Fig. 1), probably for identical reasons. Regarding gas exchange, the blood gas and MIGET results showed no effect of PEEPe, which is in apparent contradiction with the improvement in PaO2 due to a higher mean value of the perfusion distribution observed by Rossi et al. [8]. However, those favorable effects occurred at a lower PEEPe (50% of PEEPi), and disappeared when PEEPe was equal to PEEPi, which again suggests excessive PEEPe levels in at least some of our patients. These observations underline the difficulty of correctly titrating PEEPe in this context. Having said this, low levels of PEEPe (≤ 50% of PEEPi) may still be of benefit, by preventing alveolar derecruitment, as shown by the study by Rossi et al. [8].
In conclusion, the present study shows that in COPD patients undergoing controlled mechanical ventilation, with PEEPi, He/O2 can be a valuable approach to reducing dynamic hyperinflation/PEEPi, while only slightly impairing arterial oxygenation due to a reduction in the mean value of perfusion distribution. Conversely, PEEPe can prove difficult to titrate and can induce worsening of dynamic hyperinflation, the latter probably offsetting any benefit of PEEPe on arterial oxygenation. Hence in patients with severe and symptomatic dynamic hyperinflation, as can occur during the first few days of mechanical ventilation, He/O2 could prove to be a valuable approach, provided the various technical issues associated with its use are known by ICU physicians.
References
Macklem PT (1984) Hyperinflation. Am Rev Respir Dis 129:1–2
Marini JJ (1989) Should PEEP be used in airflow obstruction? Am Rev Respir Dis 140:1–3
Pepe, P Marini J (1982) Occult positive end-expiratory pressure in mechanically ventilated patients with airflow obstruction. Am Rev Respir Dis 126:166–170
Rossi A, Polese G, Brandi G, Conti G (1995) Intrinsic positive end-expiratory pressure. Intensive Care Med 21:522–536
Smith TC, Marini JJ (1988) Impact of PEEP on lung mechanics and work of breathing in severe airflow obstruction. J Appl Physiol 65:1488–1499
Tuxen D, Lane S (1987) The effects of ventilatory pattern on hyperinflation, airway pressures, and circulation in mechanical ventilation of patients with severe airflow obstruction. Am Rev Respir Dis 136:872–879
Bernasconi M, Brandolese R, Poggi R, Manzin E, Rossi A (1990) Dose-response effects and time course of effects of inhaled fenoterol on respiratory mechanics and arterial oxygen tension in mechanically ventilated patients with chronic airflow obstruction. Intensive Care Med 16:108–114
Rossi A, Santos C, Roca J, Torres A, Félez MA, Rodriguez-Roisin R (1994) Effects of PEEP on VA/Q mismatching in ventilated patients with chronic airflow obstruction. Am J Respir Crit Care Med 149:1077–1084
Tuxen D (1989) Detrimental effects of positive end-expiratory pressure during controlled mechanical ventilation of patients with severe airflow obstruction. Am Rev Respir Dis 140:5–9
Georgopoulos D, Gianoulli E, Patakas D (1993) Effects of extrinsic positive end-expiratory pressure on mechanically ventilated patients with chronic obstructive pulmonary disease and dynamic hyperinflation. Intensive Care Med 19:197–203
Baigorri F, De Monte A, Blanch L, Fernandez R, Vallés J, Mestre J, Saura P, Artigas A (1994) Hemodynamic responses to external counterbalancing of auto-positive end-expiratory pressure in mechanically ventilated patients with chronic obstructive pulmonary disease. Crit Care Med 22:1782–1791
Papamoschou D (1995) Theoretical validation of the respiratory benefits of helium-oxygen mixtures. Respir Physiol 99:183–199
Tassaux D, Jolliet P, Roeseler J, Chevrolet JC (2000) Effects of helium-oxygen on intrinsic positive end-expiratory pressure in intubated and mechanically ventilated patients with severe chronic obstructive pulmonary disease. Crit Care Med 28:2721–2728
Tassaux D, Jolliet P, Thouret JM, Roeseler J, Dorne R, Chevrolet JC (1999) Calibration of seven ICU ventilators for mechanical ventilation with helium-oxygen mixtures. Am J Respir Crit Care Med 160:22–32
Manier G, Guénard H, Castaing Y, Varène N (1983) Respiratory gas exchange under heliox breathing in COPD studied by the inert gas method. Bull Eur Physiopathol Respir 19:401–406
Thiriet M, Douguet D, Bonnet JC, Canonne C, Hatzfeld C (1979) The effect on gas mixing of a He-O2 mixture in chronic obstructive lung disease. Bull Eur Physiopathol Respir 15:1053–1068
Pauwels RA, Buist S, Calverley PMA, Jenkins CR, Hurd SS (2001) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 163:1256–1276
Jolliet P, Tassaux D, Thouret JM, Chevrolet JC (1999) Beneficial effects of helium-oxygen vs. air-oxygen non-invasive pressure support in decompensated COPD patients. Crit Care Med 27:2422–2429
Brochard L (2002) Intrinsic (or auto-) PEEP during controlled mechanical ventilation. Intensive Care Med 28:1376–1378
Wagner PD, Saltzmann HA, West JB (1974) Measurement of continuous distributions of ventilation-perfusion ratios: theory. J Appl Physiol 36:588–599
Wagner PD, Smith CM, Davies NJH, McEvoy RD, Gale GE (1985) Estimation of ventilation-perfusion inequality by inert gas elimination without arterial sampling. J Appl Physiol 59:376–383
Evans JW, Wagner PD (1977) Limits on VA/Q distributions from analysis of experimental inert gas elimination. J Appl Physiol 42:889–898
Roca J, Wagner PD (1994) Principles and information content of the multiple inert gas elimination technique. Thorax 49:815–824
Paiva M, Engel LA (1979) Pulmonary interdependence of gas transport. J Appl Physiol 47:296–305
Christopherson SK, Hlastala MP (1982) Pulmonary gas exchange during altered density gas breathing. J Appl Physiol 52:221–225
Wagner PD, Dantzker DR, Dueck R, Clausen JL, West JB (1977) Ventilation-perfusion inequality in chronic obstructive pulmonary disease. J Clin Invest 59:203–216
Torres A, Reyes A, Roca J, Wagner PD, Rodriguez-Roisin R (1989) Ventilation-perfusion mismatching in chronic obstructive pulmonary disease during ventilator weaning. Am Rev Respir Dis 140:1246–1250
Jaber S, Fodil R, Carlucci A, Boussarsar M, Pigeot J, Lemaire F, Harf A, Lofaso F, Isabey D, Brochard L (2000) Noninvasive ventilation with helium-oxygen in acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 161:1191–1200
Yang SC, Yang SP (2002) Effects of inspiratory flow waveforms on lung mechanics, gas exchange, and respiratory metabolism in COPD patients during mechanical ventilation. Chest 122:2096–2104
Guérin C, Milic-Emili J, Fournier G (2000) Effect of PEEP on work of breathing in mechanically ventilated COPD patients. Intensive Care Med 26:1207–1214
Nava S, Bruschi C, Rubini F, Palo A, Iotti G, Braschi A (1995) Respiratory response and inspiratory effort during pressure support ventilation in COPD patients. Intensive Care Med 21:871–879
Fernandez Mondejar E, Mata GV, Navarro PN, Fernandez RR, Ruiz JMT, Carazo E (1992) Increase in lung volume originated by extrinsic PEEP in patients with auto-PEEP. Intensive Care Med 18:269–273
Gay P, Rodarte J, Hubmayr R (1989) The effects of positive expiratory pressure on isovolume flow and dynamic hyperinflation in patients receiving mechanical ventilation. Am Rev Respir Dis 139:621–626
Ranieri VM, Giuliani R, Cinnella G, Pesce C, Brienza N, Ippolito EL, Pomo V, Fiore T, Gottfried SB, Brienza A (1993) Physiologic effects of positive end-expiratory pressure in patients with chronic obstructive pulmonary disease during acute ventilatory failure and controlled mechanical ventilation. Am Rev Respir Dis 147:5–13
Patel H, Yang KL (1995) The variability of intrinsic positive end-expiratory pressure in patients receiving mechanical ventilation. Crit Care Med 23:1074–1079
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jolliet, P., Watremez, C., Roeseler, J. et al. Comparative effects of helium-oxygen and external positive end-expiratory pressure on respiratory mechanics, gas exchange, and ventilation-perfusion relationships in mechanically ventilated patients with chronic obstructive pulmonary disease. Intensive Care Med 29, 1442–1450 (2003). https://doi.org/10.1007/s00134-003-1864-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-003-1864-2






