Abstract
Objective
To examine the relationship of fluid balance and weaning outcomes.
Methods
We prospectively collected demographic, physiological, daily fluid balance (measured inputs minus outputs), and weaning data from 87 mechanically ventilated patients.
Patients
We examined 87 patients, a median age of 66 years, APACHE II of 22, and performed 205 breathing trials (BT); 38 patients (44%) were successfully extubated after their first BT with minimal or no pressure support.
Results
Positive fluid balance (inputs>outputs) in the 24, 48, and 72 h and cumulatively (from hospital admission) prior to weaning were significantly greater in weaning failures than successes. Both univariate and multivariate analyses, adjusted for duration of mechanical ventilation and presence of chronic obstructive pulmonary disease, showed negative cumulative fluid balance 24 h prior to BTs (OR=2.9) and cumulative fluid balance (OR=3.4) to be independently associated with first-day weaning success. Similar relationships were demonstrated when all weaning attempts were analyzed. Negative fluid balance was as predictive of weaning outcomes as f/Vt (likelihood of success was 1.7 for patients with negative fluid balance 24 h prior to weaning and 1.2 for those with f/Vt<100 min−1 l−1). Although administration of diuretics was associated with more negative fluid balance, it was not independently associated with weaning outcomes.
Conclusions
These data suggest that fluid balance, a potentially modifiable factor, is associated with weaning outcomes. A randomized study is required to determine whether diuresis to treat positive fluid balance expedites liberation from mechanical ventilation.
Similar content being viewed by others
Introduction
Mechanical ventilation engenders significant risks to patients [1], and thus clinicians should strive to liberate patients as quickly as possible. Minimizing the duration of mechanical ventilation can be achieved by: (a) hastening repair of the pathophysiological abnormalities that cause respiratory failure, (b) recognizing readiness for liberation, and (c) preventing/minimizing complications associated with mechanical ventilation. Most research aimed at expediting liberation from invasive mechanical ventilation has centered on methods of determining readiness to breathe without the assistance of the ventilator [2, 3, 4] and without an artificial airway [5, 6]. A preponderance of evidence suggests that liberation is achieved most expeditiously if hemodynamically stable patients with sufficient oxygenation are tested daily with breathing trials and extubated when appropriate [2, 3, 4]. There is a paucity of research addressing methods of repairing patients’ pathophysiological processes and iatrogenic complications to reduce the duration of respiratory failure [7, 8].
Treatment of many critical illnesses requires volume resuscitation in the early phases of illness. Institution of mechanical ventilation itself commonly causes hypotension [9] due to reduced venous return [10] and administration of sedatives that requires administration of fluids to sufficiently refill the circulation. In a previous study we demonstrated that negative fluid balance is associated with survival in patients with septic shock [11]. It is plausible that fluid administered commonly in the initial resuscitation of critically ill patients must be retrieved before patients can be weaned successfully. In this study we hypothesized that fluid balance is associated with weaning outcomes [12].
Methods
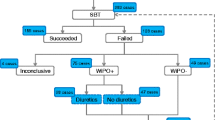
Our hospital institutional review committee approved conduct of this study. All intubated patients admitted to our medical ICU between August 2002 and March 2003, who underwent breathing trials (BT) for the purpose of considering them for a trial of extubation, were eligible for the study. In our (“open-model”) ICU weaning is guided by a nonmandatory protocol that is performed by bedside nurses, respiratory therapists, and resident trainees who are supervised by five Board-certified intensivists. Patients who are hemodynamically stable without vasoactive medications, and whose PaO2:FIO2 ratio is 120 or higher are assessed with BTs consisting of either T-piece or pressure support of 7 cmH2O or less. There is no explicit protocol, but sedatives are stopped between 0.5 and 8 h prior to BT (depending upon the sedative). The BT is terminated (and considered a failure) if patients develop: subjective distress despite attempts of bedside personnel to allay their anxiety, increments of heart rate of more than 20 beats/min, systolic blood pressure higher than 20 mmHg, respiratory rate greater than 35 breaths/min, tidal volumes above 0.3 l, or sustained pulse oximetry desaturations to more than 90% while inspiring 50% oxygen. Subjective distress (during weaning failure) may include complaints of breathlessness despite reassurance of bedside caregivers, chest pain, agitation (e.g., flailing), or altered mentation. Those who successfully complete a BT, and whose airway is judged competent (i.e., ability to expectorate and follow simple commands) are considered for a trial of endotracheal extubation. For purposes of this study “weaning success” was defined as extubation without artificial ventilation for at least 72 h. Patients who had a tracheotomy were considered successfully weaned when they remained off the ventilator for at least 72 h. Patients who failed or who were reintubated within 72 h were defined as weaning failures.
A uniform data abstraction tool was used to gather data from patients’ medical records. Demographic data including age, gender, race, comorbidities and acute physiology, and APACHE II scores on admission and on the first day of weaning were recorded. Presence of left ventricular dysfunction was documented if there was echo- or electrocardiographic evidence of left ventricular hypertrophy and/or diastolic or systolic left ventricular dysfunction cited in an echocardiogram report. On the day of each BT we noted PaO2/FIO2 ratio, prealbumin (if available), weaning parameters [ratio of respiratory frequency to tidal volume (f/Vt) measured on CPAP=5 cmH2O], respiratory system resistance (peak pressure−plateau pressure/flow), static respiratory compliance measured during assist control (Vt/plateau pressure−PEEP), and BT outcome. Use of diuretics in the 24 h prior to or during the BT and daily measured fluid inputs and outputs were recorded. Cumulative fluid balance (including fluids, medications and blood products) was defined as total inputs minus total outputs from the time of endotracheal intubation to the day of the BT. Fluid balance in the 24 h prior to the BT refers to inputs minus outputs (tallied at midnight) of the day prior to the BT.
The study enrolled 87 patients with a mean age of 63.8 years (range 20–88, median 66; see Table 1), including 57 men and 30 women. Acute Physiology and Chronic Health Evaluation (APACHE) II scores on admission ranged from 9 to 42, with a mean of 22. The primary reasons for their ICU admissions and intubation were airway protection for a variety of medical conditions (n=33), pneumonia (n=27), congestive heart failure (n=9), exacerbations of chronic obstructive lung disease (n=14), asthma (n=4), sepsis (n=5), and a variety of other causes (n=8). The 87 patients underwent a total of 205 BTs: 42 T-piece, 151 pressure support (≤7 cmH2O, with or without PEEP), and 12 both.
The main outcome of interest was weaning failure or success. Sensitivity, specificity, and likelihood ratios of predicting successful weaning using negative fluid balances were computed and compared with those obtained using the f/Vt. Risk ratios (RRs) were computed as the preferred measure of the strength of association between the predictive variables and the binary outcomes of interest. Mean values of the continuous outcome variables were compared across categories of potential predictors using analysis of variance. Comparisons of median values were made using nonparametric methods (Kruskal-Wallis and Mann-Whitney tests). For grouped data the χ2 or Fisher’s exact test was used in comparing differences in proportions between the two groups and in deriving p values. Logistic regression analyses were performed to ascertain the independent predictive potential of fluid balance, adjusting for potential confounding variables. Criteria for inclusion of variables in the logistic regression models were based on findings from prior studies, current clinical practice and evidence of an association (p<0.05) in the univariate analysis. The appropriateness of the fitted model and its predictive utility were confirmed. Summary measures of goodness of fit were evaluated using standard tests [13]. Analyses were facilitated by the use of EPIINFO 2002 and STATISTICA software packages. A p value less than 0.05 was used to determine statistical significance.
Results
First-day weaning outcomes
Thirty-eight (44%) patients were successfully extubated after their first BT. Table 1 compares characteristics of those who were successfully extubated vs. those who failed either the BT or extubation on their first day of weaning. Patients who were successfully extubated had significantly negative fluid balance in the preceding 24 h (median −625 ml vs. +242 ml, p=0.01), 48 h (median −828.50 vs. +733, p=0.01) and net negative cumulative balance (median −633 vs. +920, p=0.06) compared to those who failed. Patients with negative cumulative fluid balance were more than twice as likely to be successfully weaned than those with net positive balance (RR=2.2; 95%CI=1.3–3.8). Patients who were successful had been intubated for a shorter duration (median 2.0 vs. 3.0 days, p=0.03) and had a lower f/Vt (median 50 vs. 80 min−1 l−1, p=0.005). Similarly, the mean PaO2/FIO2 ratio was significantly higher in weaning successes (median 231 vs. 196, p=0.03). Presence of chronic obstructive pulmonary disease was also associated with first-day failure (p=0.04). There were no statistically significant differences between the two groups in prealbumin level, respiratory compliance, or the prevalence of left ventricular dysfunction. Although administration of diuretics was associated with negative fluid balance, it was not associated with weaning outcomes.
In multiple logistic regression models negative fluid balance retained its predictive potential (of success) even after adjusting for duration of mechanical ventilation and presence of chronic obstructive pulmonary disease that were also associated with outcomes in univariate analyses (OR=2.9, 95% CI=1.1–7.6, for negative fluid balance 24 h prior; OR=3.4, 95% CI=1.3–8.7, for cumulative negative balance). Although associated with weaning outcomes in univariate analysis, the f/Vt was not included in the multivariate model because rapid shallow breathing was used to identify weaning failure in our ICU (it is not an entirely independent variable). Table 2 shows sensitivities, specificities, positive predictive values, negative predictive values, and likelihood ratios of negative fluid balance and extubation success. Negative cumulative fluid balance had similar predictive characteristics as f/Vt.
All weaning attempts
There were no substantive differences when all BTs (i.e., multiple attempts for some patients who were initial failures) were analyzed; fluid balance was consistently associated with weaning outcomes when subsequent attempts were also considered (data available upon request).
Discussion
This study demonstrates that fluid balance, a potentially modifiable variable, is associated with weaning outcomes. Weaning success was most often achieved as patients’ cumulative fluid balance approached balance (i.e., between net +1 l and inputs=outputs). Although administration of diuretics was associated with achieving negative fluid balance, it was not independently associated with weaning outcomes.
When positive pressure ventilation is discontinued, venous return increases [10] and may contribute to weaning failure in some patients [14]. Since BTs were stopped when patients developed subjective distress, tachypnea, or oxygen desaturation, the precise cause of weaning failure was not determined. A retrospective case control study of patients undergoing cardiac surgical interventions [15] found a statistically significant difference in intraoperative fluid balance between patients who were successfully weaned and those who required reintubation. In addition, a relationship of weaning outcomes and fluid balance in surgical ICU patients has been reported previously in abstract form [16]. Ours is the first completed study, to our knowledge, that demonstrates an association of fluid balance and weaning outcomes in critically ill medical patients.
There are several weaknesses of the current study. First, since ours is an “open-model” ICU, weaning did not occur with a mandatory protocol. Although our clinicians and respiratory therapists are guided by the protocol presented above, it is possible that the first day of weaning was delayed beyond when patients met criteria. Practitioners’ bias could have affected the outcome if fluid balance was a criteria used by some to determine when to begin BTs. Second, the average number of BTs per patient was relatively small (just more than two per patient), and this therefore represents a group with relatively few persistent weaning failures. These results should be generalized cautiously to dissimilar ICU populations. Third, fluid balance was determined at 12 p.m. (midnight) for the previous 24 h whereas BTs occurred the following morning. Subsequent changes in fluid balance (between midnight and the time of the BT) could acutely impact weaning beyond that discernible with the recorded balance. This shortcoming is most likely to affect the relationship of 24-h fluid balance and outcome. However, the consistent association of fluid balance and weaning outcomes, irrespective of the duration of measurement, suggests a more robust association.
There were no specific study-prescribed criteria for treatment with diuretics. Care of patients in our ICU is provided by five intensivists. In the recovery phase of illness one intensivist “diureses until the blood urea nitrogen rises” while others do not share this approach. Our study does not discern whether negative fluid balance is a result of resolving pathophysiological processes that eventuate in weaning success or is a direct result of therapeutic interventions (i.e., administration of diuretics). Also, even though APACHE II scores on the day of weaning were not significantly different, it has not been well-validated for use beyond ICU admission. Therefore severity of illness and other factors not considered in our multiple logistic regression model, such as airway competence [5, 6] and sedation history [8, 17], may also affect weaning outcomes. These limitations notwithstanding, the observed relationship of fluid balance and weaning outcomes merits replication at other centers. A prospective randomized study would be required to determine whether therapeutic diuresis expedites liberation from mechanical ventilation.
References
Mutlu GM, Factor P (2000) Complications of mechanical ventilation. Respir Care Clin N Am 6:213–252
Yang KL, Tobin MJ (1991) A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med 324:1445–1450
MacIntyre NR, Cook DJ, Ely EW Jr, Epstein SK, Fink JB, Heffner JE, Hess D, Hubmayer RD, Scheinhorn DJ (2001) Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest 120:375S-95S
Epstein SK (2000) Weaning parameters. Respir Care Clin N Am 6:253–301
Khamiees M, Raju P, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA (2001) Predictors of extubation outcome in patients who have successfully completed a spontaneous breathing trial. Chest 120:1262–1270
Smina M, Salam A, Khamiees M, Gada P, Amoateng-Adjepong Y, Manthous CA (2003) Cough peak flows and extubation outcomes. Chest 124:262–268
Smyrnios NA, Connolly A, Wilson MM, Curley FJ, French CT, Heard SO, Irwin RS (2002) Effects of a multifaceted, multidisciplinary, hospital-wide quality improvement program on weaning from mechanical ventilation. Crit Care Med 30:1224–1230
Kress JP, Pohlman AS, O’Connor MF, Hall JB (2000) Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 342:1471–1477
Franklin C, Samuel J, Hu TC (1994) Life-threatening hypotension associated with emergency intubation and the initiation of mechanical ventilation. Am J Emerg Med 12:425–428
Luce JM (1984) The cardiovascular effects of mechanical ventilation and positive end-expiratory pressure. JAMA 252:807–811
Alsous F, Khamiees M, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA (2000) Negative fluid balance predicts survival in patients with septic shock: a retrospective pilot study. Chest 117:1749–1754
Upadya A, Ogbata S, Muralidharam V, Amoateng-Adjepong Y, Manthous CA (2003) Cumulative fluid balance affects weaning outcomes. Am J Respir Crit Care Med 167:A301
Hosmer DW, Lemeshow S (2000) Applied logistic regression. Wiley, New York
Lemaire F, Teboul JL, Cinotti L, Giotto G, Abrouk F, Steg G, Macquin-Mavier I, Zapol WM (1988) Acute left ventricular dysfunction during unsuccessful weaning from mechanical ventilation. Anesthesiology 69:171–179
Engoren M, Buderer NF, Zacharias A, Habib RH (1999) Variables predicting reintubation after cardiac surgical procedures. Ann Thorac Surg 67:661–665
Epstein CD, Peerless JR, Mohamed HF, Wrenn ER, Kuestner ME (2003) Relationship between fluid balance & weaning in older critically ill surgical patients. Am J Crit Care 13:273
Khamiees M, Amoateng-Adjepong Y, Manthous CA (2002) Propofol infusion is associated with a higher rapid shallow breathing index in patients preparing to wean from mechanical ventilation. Respir Care 47:150–153
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Upadya, A., Tilluckdharry, L., Muralidharan, V. et al. Fluid balance and weaning outcomes. Intensive Care Med 31, 1643–1647 (2005). https://doi.org/10.1007/s00134-005-2801-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-2801-3