Abstract
Objective
To determine the diagnostic role of soluble triggering receptor expressed on myeloid cells (sTREM)-1 in non-directed bronchial lavage fluid in ventilator-associated pneumonia (VAP).
Design
Non-directed bronchial lavage fluid and plasma were collected on alternate days in critically ill mechanically ventilated patients from the start of ventilatory support until complete weaning from the ventilator. Soluble TREM-1 levels were measured by an enzyme-linked immunosorbent assay.
Setting
A general adult medical and surgical university hospital intensive care unit.
Patients
Nine patients who developed VAP and 19 patients who did not develop VAP (controls).
Results
Plasma levels of sTREM-1 did not change significantly in either patient group. While in controls concentrations of sTREM-1 in non-directed bronchial lavage fluid did not change significantly over time, in patients who developed VAP levels of sTREM-1 in non-directed bronchial lavage fluid increased towards the diagnosis of VAP. A cut-off value for non-directed bronchial lavage fluid sTREM-1 levels of 200 pg/ml on the day of VAP had a diagnostic sensitivity of 75% and a specificity of 84%. Sensitivity increased when taking into account all sTREM-1 levels higher than 200 pg/ml from the 6-day period before the day of diagnosis that were preceded by an increase of at least 100 pg/ml (sensitivity 88%, specificity 84%).
Conclusions
Soluble TREM-1 is a potential biomarker of VAP.
Similar content being viewed by others
Introduction
The diagnosis of ventilator-associated pneumonia (VAP) remains a major challenge for intensive care unit (ICU) physicians [1, 2]. Although a presumptive clinical diagnosis of VAP is often made on the basis of presence of a combination of new radiographic infiltrates on chest radiography, fever, leukocytosis, hypoxemia and/or purulent tracheal secretions, such an approach inevitably leads to overestimation of the incidence of VAP and subsequent unwanted excessive antibiotic use [3]. Invasive or noninvasive microbiological diagnostic procedures require some form of laboratory processing that further delays a definitive diagnosis for 24–48 h, which may cause clinicians to start unneeded antibiotics while awaiting the laboratory results.
Many biological markers have been studied in an effort to improve the diagnostic procedure and assessment of prognosis in VAP such as procalcitonin [4, 5, 6], C-reactive protein [5], proinflammatory cytokines [7, 8, 9] and elements of the coagulation cascade [10]. Local levels of tumor necrosis factor (TNF)-α, interleukin (IL) 6 and plasminogen activator inhibitor 1 appear to be elevated prior to clinical diagnosis of pneumonia. However, these markers have not been evaluated as clinical biomarkers for pneumonia. The reports on procalcitonin as a biomarker have shown promising results [4, 5, 6], but these findings were not reproduced in another study [11]. Recently a new diagnostic marker for VAP has been proposed [11]. The triggering receptor expressed on myeloid cells (TREM-1) is a member of the immunoglobulin superfamily and only weakly expressed on cells in tissues from patients with noninfectious inflammatory disorders [12]. Upon invasion of bacteria or fungi tissues are infiltrated with neutrophils and monocytes that strongly express TREM-1. A soluble form of TREM-1 (sTREM-1) can be measured in various body fluids, possibly reflecting TREM-1 shed from the membranes of activated phagocytes [11, 13]. In this way levels of sTREM-1 in bronchoalveolar fluid may serve as a biomarker for pulmonary infection. One of the present authors reported increased sTREM-1 levels in bronchoalveolar fluid of patients with community-acquired or ventilator associated pneumonia [11]. It was shown that the presence of sTREM-1 by itself was more accurate than any clinical finding or laboratory value in identifying the presence of pneumonia. In that study, however, sTREM-1 levels were measured at only a single time point.
Recently we reported changes in local cytokine and procoagulant mediator levels in patients who developed VAP and compared these levels with those in patients who did not develop pneumonia during mechanical ventilation [9, 10]. The goal of this study, the preliminary findings of which have been presented previously [14], was to analyze sTREM-1 levels in non-directed bronchial lavage fluids (NBLF) and matching plasma samples from these patients. We compared the capacity of sTREM-1 to differentiate between the presence and absence of pneumonia with that of local cytokine levels on which we previously reported [9].
Material and methods
Patients
Patients are identical to those in the previous reports [9, 10]. In short, 60 critically ill patients expected to need mechanical ventilation for at least 5 days were included within 72 h of admission to the ICU. Use of immunosuppressive drugs was an exclusion criterion. Of the initial cohort 32 patients were excluded because the duration of mechanical ventilation was less than 5 days. None of the patients who were excluded developed VAP; nine of the remaining patients developed VAP after 9.6±3.0 days. Baseline characteristics are presented in Table 1.
Non-directed bronchoalveolar lavage and blood sampling
As long as patients were mechanically ventilated, each alternate day a non-directed bronchial lavage (NBL) was performed as described previously [15]. NBL was performed by instilling 20 ml sterile 0.9% saline via a standard 50-cm, 14-gauge tracheal suction catheter. For this the distal end of the catheter was introduced via the endotracheal tube and advanced until significant resistance was encountered. Immediately after instillation over 4–5 s fluid was aspirated before withdrawal of the catheter. Generally 4-8 ml fluid was recovered. Plasma samples were collected before each lavage into sterile vacutainer tubes containing heparin. NBLF)and plasma samples were kept at 4°C and processed within 1 h. Samples were centrifuged at 1,500 g for 15 min at 4°C; thereafter samples were stored at −80°C until assays were performed.
Determination of sTREM-1
Unlike previous studies in which sTREM-1 levels were measured by means of western blot [11, 13], concentrations of sTREM-1 in NBLF and plasma were determined by a recently developed sandwich enzyme-linked immunosorbent assay. In short, 96-well goat anti-mouse IgG coated plates (R&D Systems, Minneapolis, Minn., USA) were incubated overnight at room temperature with 400 ng per well mouse anti-human TREM-1 capture antibody (R&D Systems). The plates were then washed with 0.05% Tween 20 in phosphate-buffered saline. Samples and standards were diluted in phosphate-buffered saline containing 20% fetal calf serum and incubated for 2 h. Human recombinant sTREM-1 was used as the standard (R&D Systems). After 3 washes 40 ng per well biotinylated goat anti-human TREM-1 (R&D Systems) was added for 2 h at room temperature. After three washes bound sTREM-1 was detected with peroxidase-conjugated streptavidin (R&D Systems) and ortho-phenylenediamine as the substrate. The color reaction was stopped after 10 min with 2 N H2SO4, and the absorbance was read at 450 and 560 nm for wavelength correction. All measurements were performed in duplicate and in a blind fashion.
NBLF levels of tumor necrosis factor α, interleukin 1β and interleukin 6
Levels of TNF-α, IL-1β and IL-6 have been reported previously [9]. The original data were used for constructing receiver operating characteristic (ROC) curves (see below).
Diagnosis of pneumonia
Diagnosis of VAP required a combination of the clinical features of VAP together with microbiological confirmation as described previously [9, 10]. The day on which antibiotic treatment was started was retrospectively assigned as the day of diagnosis of VAP.
Statistical analysis
Data on admission for VAP patients were compared with data of control patients using Student’s t test for parametric continuous data and the χ2 test for categorical data. Data are presented for individual patients. For comparisons between patients developing VAP and control patients sTREM-1 concentrations of VAP patients were compared to the highest sTREM-1 levels of the control group recorded during the study period. ROC curves were constructed to illustrate various cut-off values of sTREM-1, TNF-α, IL-1β and IL-6. Data within each group were analyzed for changes over time using linear mixed models. In the control group this was carried out in a straightforward manner. For VAP patients two different linear mixed models were created. The first model describes changes over time in sTREM-1 levels during the 6-day period leading up to the day of diagnosis of VAP. The second model investigates changes over time after treatment was initiated. Differences at the level of p<0.05 were considered statistically significant.
Results
sTREM-1 levels in plasma and NBLF
Plasma sTREM-1 levels of neither VAP nor control patients changed in time (data not shown). From the day of ICU admission, NBLF sTREM-1 levels did not change significantly in control subjects, while local levels of sTREM-1 in VAP patients rose before the diagnosis of VAP (Fig. 1). A significant increase in sTREM-1 levels in NBLF was observed during the 6-day period until the day of VAP diagnosis (p=0.032, linear mixed model). The mean concentration of sTREM-1 increased from 155 pg/ml (95% confidence interval, CI, 0–574) to a maximum level of 894 pg/ml (499–1290) on the day on which VAP was clinically diagnosed. The greatest increase in alveolar sTREM-1 levels was observed from 2 days before until the day of VAP diagnosis. Levels on the day of VAP diagnosis were significantly higher than those 2 days before diagnosis (p=0.015, linear mixed model). Thereafter sTREM-1 levels rapidly decreased (p=0.015, linear mixed model). All patients responded well to antibiotic therapy.
Time-course of soluble triggering receptor expressed on myeloid cells (sTREM-1) in non-directed bronchial lavage fluid of individual patients. Left Time course in control patients (n=19); day 0 start of mechanical ventilation. Right levels in patients developing VAP (n=9); day 0 day of diagnosis of ventilator-associated pneumonia
sTREM-1 as a discriminative marker for VAP
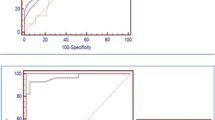
We investigated whether concentrations of sTREM-1 in NBLF discriminate VAP patients from control patients. For each control patient the highest sTREM-1 value recorded during the study period was used to compare with the levels of sTREM-1 from the VAP patients on day of diagnosis (model 1). A level of at least 200 pg/ml was detected in six of the eight VAP patients on the day of clinical diagnosis (sensitivity of 75%). (Unfortunately, one sample of a VAP patient was missing from the day of onset of pneumonia). A level of at least 200 pg/ml was detected in 3 of the 19 control patients (specificity 84%). A ROC curve was constructed to show the capacity of sTREM-1 in discriminating patients with VAP from the controls (Fig. 2a). The area under the ROC curve was 0.83 (95% CI 0.65–1.00, p=0.008).
Receiver-operating-characteristic curves for various cut-off levels of soluble triggering receptor expression on myeloid cells (sTREM-1), TNF-α, IL-1β and IL-6 in non-directed bronchoalveolar lavage fluid. a ROC curve for sTREM-1 levels on the day of diagnosis (model 1, see text for details). b ROC curve for sTREM-1 levels during the 6-day period before VAP diagnosis (model 2). c ROC curve for increases in sTREM-1 level during the 6-day period before VAP diagnosis (model 3). d, e, f ROC curve for levels of TNF-α (left), IL-1β (middle) and IL-6 (left) during the 6-day period before VAP diagnosis. In all graphs the diagonal line represents the reference line
When all sTREM-1 levels during the 6 days prior to VAP diagnosis until the day of VAP diagnosis were considered (model 2), a level of 200 pg/ml was detected in all but one VAP patient (sensitivity 88%) and in 3 of the 19 control patients (specificity 84%). The area under the ROC curve was 0.86 (95% CI 0.71–1.00, p=0.003; Fig. 2b).
We then investigated whether an increase in sTREM-1 during the 6 days prior to VAP diagnosis was prognostic for VAP (model 3). An increase of 100 pg/ml or greater in between-measurements was predictive of pulmonary infection in seven of the eight VAP patients (sensitivity of 88%). Five of 19 controls were falsely diagnosed for having VAP (specificity 74%) The area under the ROC curve was 0.88 (95% CI 0.75–1.0, p=0.002; Fig. 2c).
If the two criteria were combined (positive if level of 200 pg/ml was preceded by an increase of at least 100 pg/ml during the 6-day period before VAP, model 4), three control patients were falsely diagnosed (specificity 84%), while one VAP patient was not detected (sensitivity 88%).
Comparison with local levels of TNF-α, IL-1β and IL-6
We compared the capability of sTREM-1 in differentiating presence of pneumonia with that of TNF-α, IL-1β and IL-6. For all analyses, the highest levels during the 6 days prior to VAP diagnosis were used. For TNF-α a cut-off value of 50 pg/ml resulted in a sensitivity of 78% and a specificity of 58%. The area under the ROC curve for TNF-α was 0.64 (95% CI 0.40–0.87, p=0.26; Fig. 2d). Similar results were obtained with IL-1β. A cut-off value of 1000 pg/ml for IL-1β resulted in a sensitivity of 67% and a specificity of 68%. The area under the ROC curve was 0.66 (95% CI 0.42–0.89, p=0.19; Fig. 2e). Better results were obtained with IL-6. A cut-off value of 1000 pg/ml for IL-6 resulted in a sensitivity of 89% and a specificity of 58%. The area under the ROC curve was 0.74 (95% CI 0.54–0.95, p=0.04; Fig. 2f).
Discussion
Diagnosing VAP remains a major challenge, and antimicrobial treatment is often started in patients without infection due to diagnostic uncertainty [16]. Previous work has shown that sTREM-1 may serve as a biomarker for pneumonia [11]. In that study, however, levels of sTREM-1 were measured at a single time point (i.e., when patients had developed clinical signs of pneumonia), and it remained unclear how sTREM-1 levels rise in time before the diagnosis of VAP. The present investigation confirms and extends the previous results: local sTREM-1 levels rise significantly before the diagnosis of VAP. Combining changes in local sTREM-1 levels over the days previous to VAP with the sTREM-1 levels on the day of VAP improved the diagnostic yield. During the 6-day period before VAP a cut-off value for NBLF sTREM-1 levels of 200 pg/ml preceded by an increase of at least 100 pg/ml resulted in the highest sensitivity and specificity.
Several limitations of the present study must be mentioned. One is the way in which pneumonia was diagnosed. The NBL method has been proposed for recognition of VAP by several authors [15, 17]. However, overall concordance rate with bronchoscopic methods is estimated to be only 80% [16]. Therefore, although the latest recommendations for diagnosis were used, it is possible that some patients of the control group were misdiagnosed as having no pneumonia. However, all of the control patients recovered without further antibiotic treatment.
One important sample was missing. In the present analysis NBLF sTREM-1 level on the day of VAP was missing for one patient. Two days before diagnosis this concentration was low, 19 pg/ml; 2 days after the diagnosis of VAP sTREM-1 concentration was high, 309 pg/ml; thus we found an increase in sTREM-1 concentration when comparing 2 days before with 2 days after the diagnosis. In every patient the sTREM-1 concentration decreased after initiation of antibiotic treatment on the day of diagnosis. This suggests that the sTREM-1 concentration was higher than 309 pg/ml on the day of diagnosis. However, to prevent misleading results, we did not replace the missing sample in our analysis.
In the present study local sTREM-1 levels quickly declined after initiation of antibiotic treatment. Of importance, all VAP patients responded well to antibiotic therapy. These findings are in line with those of Gibot et al. [18] showing a progressive decline in systemic levels of sTREM-1 in surviving septic patients. It remains uncertain, however, whether levels of sTREM-1 may be used as an indication of inadequate antibiotic treatment. Unfortunately, this study did not include any patient who did not respond to the initial antibiotic therapy. Further investigation is clearly necessary to make clear whether local sTREM-1 levels may be useful in monitoring antimicrobial therapy in mechanically ventilated patients.
High systemic levels of sTREM-1 are indicative of sepsis. Patients with systemic infection have increased plasma concentrations of sTREM-1 [13]. In addition, septic patients are prone to develop acute lung injury [19], a condition characterized by increased permeability of the alveolocapillary membrane [20]. In theory, systemic proteins may enter the alveolar compartment thereby leading to falsely high local sTREM-1 levels. Although we found no relationship with development of VAP and systemic sTREM-1 levels in the present analysis, increases in sTREM-1 in NBLF of septic patients are therefore to be interpreted with caution. Future research is needed to address this issue adequately.
Advantages of measuring sTREM-1 levels in NBLF as a biomarker for pneumonia are obvious: the method of NBL is generally considered to be safe, quick and inexpensive [21]. Results of laboratory testing are available within hours instead of 24–48 h for microbiology cultures to become positive. Knowing the hospital-associated pattern of infection, antibiotic treatment can be started soon after finding an elevated sTREM-1 level.
Although cytokines were measured in both plasma and NBLF, we chose not to evaluate plasma concentrations as diagnostic parameters since there was no difference in levels of cytokines in plasma between the VAP patients and the control group. We compared the capability of sTREM-1 to identify VAP patients from the controls with TNF, IL-1 and IL-6. These cytokines were not as capable as sTREM-1 of identifying patients with VAP from those without. These findings are in agreement with earlier findings of other studies [7, 8] and are not surprising since cytokines are not specific for infection. After a microbial challenge, neutrophils and macrophages are attracted to the site of infection and become activated. There they release multiple endogenous mediators to initiate innate immunity. However, especially in critically ill patients, numerous noninfectious stimuli may evoke similar inflammatory reactions. Bouchon et al. [12] showed that TREM-1 is upregulated on neutrophils and monocytes only when challenged by bacterial or fungal agents in contrast to the same cells in conditions as ulcerative colitis or psoriasis.
Our findings suggest a cut-off value of 200 pg/ml preceded by an increase of 100 pg/ml in NBLF to differentiate patients with VAP from patients without VAP. This is in contrast with the findings of Gibot et al. [11] who found a cut-off value of 5 pg/ml. This is explained by the fact that in our study an enzyme-linked immunosorbent assay was used instead of western blot techniques to quantify the amount of sTREM-1 in our samples, along with the use of a different antibody. Different standards were used probably reflecting the difference in concentrations.
In conclusion, our results confirm the usefulness of sTREM-1 as a biological marker for pneumonia. Increases in alveolar levels of sTREM-1 are diagnostic for development of pneumonia. A cut-off value of 200 pg/ml preceded by an increase of 100 pg/ml was diagnostic for pneumonia in ventilated patients.
References
Chastre J, Combes A, Luyt CE (2005) The invasive (quantative) diagnosis of ventilator-associated pneumonia. Respir Care 50:797–812
Niederman MS (2005) The clinical diagnosis of ventilator-associated pneumonia. Respir Care 50:788–796
Fagon JY, Chastre J, Hance AJ, Domart Y, Trouillet JL, Gibert C (1993) Evaluation of clinical judgment in the identification and treatment of nosocomial pneumonia in ventilated patients. Chest 103:547–553
Duflo F, Debon R, Monneret G, Bienvenu J, Chassard D, Allaouchiche B (2002) Alveolar and serum procalcitonin: diagnostic and prognostic value in ventilator-associated pneumonia. Anesthesiology 96:74–79
Brunkhorst FM, Al Nawas B, Krummenauer F, Forycki ZF, Shah PM (2002) Procalcitonin, C-reactive protein and APACHE II score for risk evaluation in patients with severe pneumonia. Clin Microbiol Infect 8:93–100
Luyt CE, Guerin V, Combes A, Trouillet JL, Ayed SB, Bernard M, Gibert C, Chastre J (2005) Procalcitonin kinetics as a prognostic marker of ventilator-associated pneumonia. Am J Respir Crit Care Med 171:48–53
Monton C, Torres A, El Ebiary M, Filella X, Xaubet A, de la Bellacasa JP (1999) Cytokine expression in severe pneumonia: a bronchoalveolar lavage study. Crit Care Med 27:1745–1753
Wu CL, Lee YL, Chang KM, Chang GC, King SL, Chiang CD, Niederman MS (2003) Bronchoalveolar interleukin-1 beta: a marker of bacterial burden in mechanically ventilated patients with community-acquired pneumonia. Crit Care Med 31:812–817
Millo JL, Schultz MJ, Williams C, Weverling GJ, Ringrose T, Mackinlay CI, van der PT, Garrard CS (2004) Compartmentalisation of cytokines and cytokine inhibitors in ventilator-associated pneumonia. Intensive Care Med 30:68–74
Schultz MJ, Millo J, Levi M, Hack CE, Weverling GJ, Garrard CS, van der PT (2004) Local activation of coagulation and inhibition of fibrinolysis in the lung during ventilator associated pneumonia. Thorax 59:130–135
Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert PE (2004) Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med 350:451–458
Bouchon A, Facchetti F, Weigand MA, Colonna M (2001) TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 410:1103–1107
Gibot S, Kolopp-Sarda MN, Bene MC, Cravoisy A, Levy B, Faure GC, Bollaert PE (2004) Plasma level of a triggering receptor expressed on myeloid cells-1: its diagnostic accuracy in patients with suspected sepsis. Ann Intern Med 141:9–15
Determann RM, Millo JL, Gibot S, Vroom MB, Garrard CS, Schultz MJ (2005) Serial changes in soluble triggering receptor expressed on myeloid cells in the lung during ventilator associated pneumonia. Crit Care 9 [Suppl 1]:P175
A’Court CH, Garrard CS, Crook D, Bowler I, Conlon C, Peto T, Anderson E (1993) Microbiological lung surveillance in mechanically ventilated patients, using non-directed bronchial lavage and quantitative culture. Q J Med 86:635–648
Chastre J, Fagon JY (2002) Ventilator-associated pneumonia. Am J Respir Crit Care Med 165:867–903
Kollef MH, Bock KR, Richards RD, Hearns ML (1995) The safety and diagnostic accuracy of minibronchoalveolar lavage in patients with suspected ventilator-associated pneumonia. Ann Intern Med 122:743–748
Gibot S, Cravoisy A, Kolopp-Sarda MN, Bene MC, Faure G, Bollaert PE, Levy B (2005) Time-course of sTREM (soluble triggering receptor expressed on myeloid cells)-1, procalcitonin, and C-reactive protein plasma concentrations during sepsis. Crit Care Med 33:792–796
Doyle RL, Szaflarski N, Modin GW, Wiener-Kronish JP, Matthay MA (1995) Identification of patients with acute lung injury. Predictors of mortality. Am J Respir Crit Care Med 152:1818–1824
Ware LB, Matthay MA (2000) The acute respiratory distress syndrome. N Engl J Med 342:1334–1349
Perkins GD, Chatterjee S, Giles S, McAuley DF, Quinton S, Thickett DR, Gao F (2005) Safety and tolerability of nonbronchoscopic lavage in ARDS. Chest 127:1358–1363
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Determann, R.M., Millo, J.L., Gibot, S. et al. Serial changes in soluble triggering receptor expressed on myeloid cells in the lung during development of ventilator-associated pneumonia. Intensive Care Med 31, 1495–1500 (2005). https://doi.org/10.1007/s00134-005-2818-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-2818-7