Abstract
Objectives
To evaluate whether PEEP affects intrapulmonary alveolar edema liquid movement and alveolar permeability to proteins during high volume ventilation.
Design and setting
Experimental study in an animal research laboratory.
Subjects
46 male Wistar rats.
Interventions
A 99mTc-labeled albumin solution was instilled in a distal airway to produce a zone of alveolar flooding. Conventional ventilation (CV) was applied for 30 min followed by various ventilation strategies for 3 h: CV, spontaneous breathing, and high volume ventilation with different PEEP levels (0, 6, and 8 cmH2O) and different tidal volumes. Dispersion of the instilled liquid and systemic leakage of 99mTc-albumin from the lungs were studied by scintigraphy.
Measurements and results
The instillation protocol produced a zone of alveolar flooding that stayed localized during CV or spontaneous breathing. High volume ventilation dispersed alveolar liquid in the lungs. This dispersion was prevented by PEEP even when tidal volume was the same and thus end-inspiratory pressure higher. High volume ventilation resulted in the leakage of instilled 99mTc-albumin from the lungs. This increase in alveolar albumin permeability was reduced by PEEP. Albumin permeability was more affected by the amplitude of tidal excursions than by overall lung distension.
Conclusions
PEEP prevents the dispersion of alveolar edema liquid in the lungs and lessens the increase in alveolar albumin permeability due to high volume ventilation.
Similar content being viewed by others
Introduction
Ventilator-induced lung injury (VILI) is an experimental concept that was first described as a consequence of lung overdistension but may also result from ventilation at low lung volume in surfactant depleted/injured lungs [1]. The clinical relevance of this concept was highlighted by the Acute Respiratory Distress Syndrome (ARDS) Network trial [2] that showed 22% reduction of mortality in patients with ARDS when the mechanical stress applied to the lungs was lessened by a reduction in tidal volume (VT). Application of positive end expiratory pressure (PEEP) may also lessen lung injury during ventilation at low lung volume [3] by avoidance of lung collapse or airway closure. Mechanical ventilation may favor intrapulmonary and systemic dissemination of sepsis and inflammation during bacterial pneumonia. It has been speculated that dissemination risk and severity depend on the occurrence of concomitant VILI [4, 5, 6]. Nahum et al. [5] found more positive blood cultures when dogs intratracheally instilled with Escherichia coli were ventilated with high VT and low PEEP rather than with low VT and high PEEP. We extended these findings to a model of unilateral Pseudomonas aeruginosa pneumonia in rats [4]. We found that ventilation with high PEEP reduced dissemination of bacteria to the contralateral lung and prevented systemic sepsis.
Understanding these observations require better knowledge of how ventilation interacts with the liquid present in airspaces. We have previously shown that ventilation above 20–25 cmH2O end-inspiratory pressure (Pei) and no PEEP dispersed alveolar edema liquid in the lungs and increased protein leakage from airspaces in rats [7]. We hypothesized that PEEP would prevent alveolar edema liquid dispersion and reduce the increase in alveolar permeability due to higher Pei ventilation modalities. Partial results of this study were presented in abstract form at the 2006 meeting of the ATS.
Methods
Animals
All experiments were conducted on male Wistar rats (weighting 281 ± 22 g; Harlan, Gannat, France) in compliance with the recommendations for laboratory animal research of the European Union and the French Ministry of Agriculture. Rats were anesthetized by intraperitoneal injection of pentobarbital (75 and 40 mg/kg 2 h later; Sigma, Saint-Quentin Fallavier, France) and remained deeply anesthetized for 4 h. They were tracheostomized and ventilated with a rodent Harvard volume ventilator (Ealing, Courtaboeuf, France).
Localized alveolar edema
99mTc-labeled albumin was prepared using a commercial kit (Vasculocis; Cis Bio International, Gif sur Yvette, France). Paper chromatography using methanol as a solvent [8] was performed to verify the amount of free 99mTc (0.03 ± 0.08%) and the stability of 99mTc binding to albumin in the final solution. Osmolarity of the 99mTc solution was made twice that of plasma adding mannitol (120 mg/ml). This solution was supplemented with bovine serum albumin (80 mg/ml), and Na+ transport inhibitors (1 mM amiloride and 1 mM phloridzin) to reduce its absorption by alveolar/airway epithelium. The 99mTc-labeled albumin solution (500 μCi in 250 μl) was slowly instilled in a distal airway after a short period of ventilation with FIO2 of 1. The instilled volume was much less than functional residual capacity, that is, about 3 ml in 300 g Wistar rats [9]. This protocol produced a localized zone of alveolar flooding [7].
Scintigraphic imaging
Acquisition was performed in the planar mode with a small γ-camera (γ Imager, Biospace, Paris, France). Acquisition window was 114–157 KeV. Each experiment lasted 210 min without interruption. A dedicated collimator was used. The decay of 99mTc activity was corrected.
Ventilation strategies
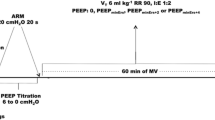
Pei was measured with a piezoelectric transducer (AST, Vanves, France) connected to the tracheal cannula. Conventional ventilation (CV) was applied for 30 min, followed by various ventilation strategies for 3 h. Control groups consisted of spontaneous breathing (SB, n = 8) and CV in ventilation with 8 ml/kg VT, 2 cmH2O PEEP, respiration rate (RR) 70/min and FIO2 1 (n = 10). Three other groups were ventilated such that (plateau) Pei was approx. 30 cmH2O, with three different levels of PEEP: 0, 6, and 8 cmH2O. Therefore these ventilation modalities were: zero PEEP (ZEEP) and high VT (HV1ZEEP; VT = 29 ml/kg, RR = 18/min, n = 8), 6 cmH2O PEEP and a lower VT (HV2PEEP6; VT 24 ml/kg, RR 24/min, n = 6), 8 cmH2O PEEP and low VT (LVPEEP 8, VT = 8 ml/kg; RR = 70/min, n = 8). Another group was ventilated with the same VT as HV2PEEP 6 but without PEEP (HV2ZEEP; VT 24 ml/kg, RR 24/min, n = 8) to better determine the effect of varying VT and/or PEEP. The latter ventilation modality resulted in Pei of 23.5 cmH2O. Respiratory rate was adjusted so that ventilation/min was the same in all groups. Ventilation strategies are summarized in Table 1.
Data analysis
Pulmonary and systemic dispersions of the tracer were studied. Regions of interest (Gamma-vision+, Biospace, Paris, France) were drawn around initial focus (“edema”, ROIE), the apex of the same lung (“apex”, ROIA), the opposite lung (“contralateral”, ROICL), and a ROI including the whole cardiopulmonary region (“total”, ROIT). Activity was integrated in each ROI over 150-s steps, expressed as percentage being divided by concurrent total, i.e., ROIT, activity. We have previously shown [7] that the clearance of 99mTc-labeled albumin instilled in rat airspaces follows a two-phase exponential decay. The initial decay slope, which is proportional to alveolar permeability-surface area product to albumin (PSA), was evaluated by linear regression [7]. PSA is the product of this initial slope and of alveolar liquid volume, about 0.5 ml, because we instilled 250 μl of a ×2 hypertonic solution.
Statistical analysis
Results are expressed as mean ± SEM. Comparisons between groups were made by anova and Bonferroni's post-hoc test or the Kruskal–Wallis test and the Dunn post-hoc test. Differences at the level of p < 0.05 were considered statistically significant (Prism, GraphPad, San Diego, Calif., USA).
Results
End-inspiratory pressure during ventilation
Figure 1 shows Pei values at t 30 and t 210. Pei was steady (mean 15.1 ± 0.55 cmH2O) in CV controls. Pei at t 30 was by design significantly higher in LVPEEP8, HV1ZEEP, HV2PEEP6, and HV2ZEEP groups (p < 0.001) than in controls. Pei increased significantly during ventilation in the HV1ZEEP group (30.1 ± 0.91 at t 30 and 42.5 ± 2.89 cmH2O at t 210, p < 0.001) and in the HV2ZEEP group (23.5 ± 0.65 at t 30 and 29.2 ± 1.03 cmH2O at t 210, p < 0.001).
Intrapulmonary dispersion
Figure 2a shows representative images obtained in a rat of the group CV. The alveolar edema remained localized and stable during 210 min. By contrast, after HV1ZEEP (applied at t 30; Fig. 2b) there was an almost immediate homo- and contralateral dispersion of the tracer. Application of PEEP abrogated this contralateral dispersion (see HV2PEEP 6 group, Fig. 2c).
Examples of scintigraphy images integrating the 15 min following instillation (t 0–t 15, left panels) and in the last 15 min of the experiment (t 195–t 200, right panels). Left panels Focalized localization of the tracer in the left lung. In CV group (a) the tracer remained remarkably confined in the initial zone; there was no contralateral and slight homolateral dissemination. HV1ZEEP ventilation (b) induced strong homo- and contralateral dispersion of the tracer and systemic leakage, as attested by the evident decrease in overall activity. HV2PEEP 6 ventilation (c) induced systemic, but not contralateral, dissemination of the tracer
As changes in the different ROIs activities relative to that of ROIT were almost linear between the time of instillation (t 0) and t 30, t 30 and t 60, t 60 and t 210 (see example in Fig. 3), data are shown at these times only, for the sake of simplicity. Activity in ROIE did not vary appreciably between t 30 and t 210 in CV or SB groups (Fig. 4a). An almost immediate decrease in ROIE activity was observed between t 30 and t 60 in HV1ZEEP and HV2ZEEP groups (p < 0.001 and p < 0.01, respectively) that was almost completely abolished by PEEP application (changes did not reach significance). The tracer did not disperse to the apex, as no significant change in ROIA/ROIT activity was observed.
Activity changes in the four ROIs expressed as proportion of initial total activity. HV1ZEEP induced an almost immediate decrease in activity in ROIE and ROIT and a dramatic increase in ROICL. Changes in ROIT activity displayed a two phase's exponential decay; activity decreased fastly between t 30 and t 60 and more slowly between t 60 and t 210
a Activity in ROIE relative to that in ROIT did not vary significantly between t 30 and t 210 in CV and HV2PEEP 6 groups. Activity significantly decreased in HV1ZEEP and HV2ZEEP groups between t 30 and t 60 (p < 0.001 and p < 0.01, respectively) and in HV1ZEEP group between t 60 and t 210 (p < 0.05). b Activity in ROICL relative to that in ROIT increased significantly between t 30 and t 60 in HV1ZEEP, HV2ZEEP (p < 0.001), and LVPEEP 8 groups (p < 0.05). This increase in activity remained significant between t 60 and t 210 in HV1ZEEP and HV2ZEEP groups (p < 0.001 and p < 0.01, respectively)
The increase in contralateral lung 99mTc-labeled albumin activity mirrored that in the instilled lung (Fig. 4b). Activity in ROICL increased significantly between t 30 and t 60 (p < 0.001) and between t 60 and t 210 in HV1ZEEP and HV2ZEEP groups (p < 0.001 and p < 0.01, respectively). A slight increase in activity was observed in the LVPEEP 8 group between t 30 and t 60 (p < 0.05), reflecting a very limited tracer movement.
Systemic albumin leakage
Figure 5 shows PSA for all ventilation strategies. In the CV group PSA was 6.2×10−3 ± 6.63×10−3 ml/h (the initial slope was 2.0×10−2/min which corresponded to a clearance rate of 99mTc-labeled albumin of 1.2%/h). PSA was similar during SB but was significantly higher during HV1ZEEP, HV2PEEP 6, and HV2ZEEP (p < 0.001) and in HV1ZEEP than during HV2PEEP 6 and HV2ZEEP (p < 0.001). This albumin leakage did not increase noticeably blood 99mTc activity, as no signal (data not shown but this is seen in from Fig. 2b and 2c) was measured in a ROI drawn over the liver, as previously observed [7]. It is thus unlikely that the 99mTc-labeled albumin present in the systemic circulation participated in the increase in contralateral lung activity. There was a trend toward an increase in PSA in the LVPEEP 8 group, but this did not reach statistical significance (0.1 > p > 0.05). There was no significant correlation between intrapulmonary 99mTc-labeled albumin redistribution (ΔROICL/h) and 99mTc-labeled albumin leakage from airspaces (ΔROIT/h) in HV1ZEEP and HV2ZEEP groups (R 2 = 0.05, NS).
Discussion
This study shows that PEEP may affect the dispersion of a zone of alveolar flooding and affect alveolar permeability to albumin: (a) High volume ventilation with no PEEP promoted contralateral dispersion of the liquid contained in a zone of alveolar flooding. (b) This dispersion was prevented by PEEP. (c) The increase in alveolar permeability to albumin due to high volume ventilation was lessened by PEEP.
Pulmonary dispersion of the tracer
We have previously shown [7] that our protocol produces a stable zone of alveolar flooding, much of the alveolar flooding coming from the circulation as during actual pulmonary edema. Hypertonic solutions in this range are not injurious [10]. This was further attested by the low systemic leakage of 99mTc-labeled albumin observed during SB or CV. Recruitment of this flooded zone by ventilation was demonstrated by computed tomography imaging [7].
Ventilation with a high VT and no PEEP (HV1ZEEP, HV2ZEEP) dispersed the labeled alveolar liquid by contrast to CV, SB or VT resulting in a Pei less or equal to 20 cmH2O [7]. Ventilation at 20 cmH2O Pei and no PEEP resulted in a VT of 14 ml/kg, a modality intermediary between CV and HV2. HV is of course never used in the clinical situation, but the inhomogeneity of ventilation distribution in patients with acute lung injury together with the “baby lung” effect [11] may lead to localized overventilation and overinflation. Ventilation with a similarly high VT is usual to mimic this situation [12, 13, 14].
Contralateral liquid dispersion began almost immediately after high VT ventilation was started (Fig. 3). Thus it can be speculated that this dispersion may be the consequence of a convective movement induced by ventilation [15], but it may also be the consequence of the compression of liquid filled zones by contiguous recruited units. Aerated lung zones may not have emptied as if there were no liquid in adjacent zones, because their conducting airways may have been obstructed earlier during expiration by liquid menisci (gas trapping) due to the back and forth movement of this liquid in airways. High flow rate is unlikely to be the only determinant of this dispersion. Peak flow rate (that is roughly proportional to Pei assuming respiratory system mechanical properties were similar in all rats) was about the same in HV1ZEEP and HV2PEEP 6 group; however, liquid dispersion was observed in the former but not in the latter. PEEP may have prevented dispersion by avoiding lung collapse and stabilizing edema fluid in the distal airways. This displacement of alveolar liquid by high VT ventilation in the absence of PEEP helps explain the intrapulmonary dissemination of bacteria that we observed in a model of unilateral P. aeruginosa pneumonia [4] during a similar ventilation modality. In the present study contralateral dispersion was abolished by PEEP application, regardless of whether VT (HV2PEEP 6 vs. HV2ZEEP) or Pei (LVPEEP vs. HV1ZEEP, HV1ZEEP vs. HV2PEEP 6) was the same. It is worth noting that PEEP had the same effect during a real, organized, pneumonia, as it prevented contralateral seeding in our model of unilateral P. aeruginosa pneumonia in rats [4].
Airspace albumin leakage
Albumin is passively absorbed from airspaces through the paracellular pathway according to its concentration gradient [16]. Leakage of 99mTc-labeled albumin from airspaces was low during CV (as well as during SB); albumin clearance being about 1.2%/h was in keeping with previous data [7, 17]. Static inflation at 40 cmH2O airway pressure did not significantly increase alveolar albumin permeability [18]. Stretching a cultured alveolar epithelium to a magnitude corresponding approx. to strains experienced in vivo at 100% total lung capacity (36% increase in surface area) produced a significant increase in permeability, whereas no alteration was observed for 12% and 25% changes in surface area [19]. Furthermore, cyclic changes between 0% to 50% produced more cell death than changes between 25% and 50% surface area [20]. The absence of a significant systemic leakage of alveolar albumin in the LVPEEP 8 group is in keeping with these observations. However, systemic leakage was significant in HV1ZEEP, HV2PEEP 6 and HV2ZEEP despite equivalent (HV1ZEEP, HV2PEEP 6) or even lower (HV2ZEEP) Pei. We have previously reported that ventilation with high VT induced a pressure-dependent increase in alveolar protein permeability when Pei was higher than 20 cmH2O in rats [7]. The present study confirms this observation. The two-exponential shape of albumin disappearance from the lungs may be due to the presence of an intermediate, interstitial compartment [7]. The later development of pulmonary edema during high-volume ventilation may have increased the back-flux of labeled albumin from this interstitial compartment to airspaces, making it impossible to calculate an unbiased permeability value. Alveolar albumin permeability was thus calculated from the first data obtained after increasing VT, before any back-flux was significant. PSA was higher during HV1ZEEP than during HV2ZEEP ventilation (p < 0.001). Interestingly, PSA was also significantly higher during HV1ZEEP than during HV2PEEP 6 ventilation despite equivalent Pei (approx. 30 cmH2O), suggesting that other mechanisms than overall lung inflation were involved. Intrapulmonary dispersion might have contributed to this systemic leakage by increasing exchange surface area. However, some albumin leakage was also observed in the HV2PEEP 6 group despite the absence of significant intrapulmonary redistribution, and no correlation was found between albumin leakage and the importance of intrapulmonary redistribution in the HV1ZEEP and HV2ZEEP groups. These observations suggest that higher than usual tidal changes in surface area in the flooded zone contributed to this increase in permeability, with, but more likely without, (considering that this increase was almost immediate) production of cell lesions. PEEP prevented the development of epithelial lesions during high VT ventilation [21], and keeping the lung open may reduce the shear stress associated with the repeated opening of collapsed peripheral units or the movement of fluid in small airways, thus possibly reducing VILI [22, 23, 24]. This is the first time, to our knowledge, that an effect of the amplitude of tidal excursions on alveolar permeability is described. High flow rates, however, are known to affect microvascular permeability in isolated lungs [25]. Uneven ventilation distribution during acute lung injury [11] may produce by places higher than expected flow rates and ventilation that increases alveolar permeability to proteins. It has been shown that patients with acute lung injury unable to increase protein concentration in pulmonary edema fluid had poorer outcome [26]. An increase in alveolar epithelial permeability to proteins may contribute to decrease liquid clearance and limit the increase in alveolar protein concentration, although alveolar edema liquid is cleared by places. Ventilation inhomogeneity may have been an unnoticed negative prognostic factor in these patients. This could have been facilitated by a rather high VT, as 46% of the patients with submaximal or impaired clearance were ventilated with more than 12 ml/kg VT.
In conclusion, this study shows that tidal volume changes may affect intrapulmonary alveolar edema liquid movement and increase alveolar permeability to albumin. PEEP prevents the intrapulmonary redistribution of edema liquid and reduces alveolar permeability to albumin. This effect of PEEP may account for the lessening of sepsis and inflammation dissemination observed during ventilation of rats with bacterial pneumonia.
References
Dreyfuss D, Saumon G (1998) Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 157:294–323
Acute Respiratory Distress Syndrome Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Muscedere JG, Mullen JB, Gan K, Slutsky AS (1994) Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 149:1327–1334
Schortgen F, Bouadma L, Joly-Guillou ML, Ricard JD, Dreyfuss D, Saumon G (2004) Infectious and inflammatory dissemination are affected by ventilation strategy in rats with unilateral pneumonia. Intensive Care Med 30:693–701
Nahum A, Hoyt J, Schmitz L, Moody J, Shapiro R, Marini JJ (1997) Effect of mechanical ventilation strategy on dissemination of intratracheally instilled Escherichia coli in dogs. Crit Care Med 25:1733–1743
Murphy DB, Cregg N, Tremblay L, Engelberts D, Laffey JG, Slutsky AS, Romaschin A, Kavanagh BP (2000) Adverse ventilatory strategy causes pulmonary-to-systemic translocation of endotoxin. Am J Respir Crit Care Med 162:27–33
Prost N de, Dreyfuss D, Saumon G (2007) Evaluation of two-way protein fluxes across the alveolo-capillary membrane by scintigraphy in rats: effect of lung inflation. J Appl Physiol 102:794–802
Dekker BG, Arts CJ, De Ligny CL (1982) Gel-chromatographic analysis of 99mTc-labeled human serum albumin prepared with Sn (II) as the reductant. Int J Appl Radiat Isot 33:1351–1357
Fisarkova B, Vizek M (2003) Hyperoxia prevents carrageenan-induced enlargement of functional residual lung capacity in rats. Physiol Res 52:763–766
Cohen DS, Matthay MA, Cogan MG, Murray JF (1992) Pulmonary edema associated with salt water near-drowning: new insights. Am Rev Respir Dis 146:794–796
Gattinoni L, Pesenti A (2005) The concept of “baby lung”. Intensive Care Med 31:776–784
Ogawa EN, Ishizaka A, Tasaka S, Koh H, Ueno H, Amaya F, Ebina M, Yamada S, Funakoshi Y, Soejima J, Moriyama K, Kotani T, Hashimoto S, Morisaki H, Abraham E, Takeda J (2006) Contribution of High-Mobility Group Box-1 to the Development of Ventilator-induced Lung Injury. Am J Respir Crit Care Med 174:400–407
Frank JA, Pittet JF, Lee H, Godzich M, Matthay MA (2003) High tidal volume ventilation induces NOS2 and impairs cAMP-dependent air space fluid clearance. Am J Physiol Lung Cell Mol Physiol 284:L791–L798
Frank JA, Wray CM, McAuley DF, Schwendener R, Matthay MA (2006) Alveolar macrophages contribute to alveolar barrier dysfunction in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 291:L1191–L1198
Wilson TA, Anafi RC, Hubmayr RD (2001) Mechanics of edematous lungs. J Appl Physiol 90:2088–2093
Hastings RH, Folkesson HG, Matthay MA (2004) Mechanisms of alveolar protein clearance in the intact lung. Am J Physiol Lung Cell Mol Physiol 286:L679–L689
Berthiaume Y, Albertine KH, Grady M, Fick G, Matthay MA (1989) Protein clearance from the air spaces and lungs of unanesthetized sheep over 144 h. J Appl Physiol 67:1887–1897
Egan EA (1982) Lung inflation, lung solute permeability, and alveolar edema. J Appl Physiol 53:121–125
Cavanaugh KJ, Cohen TS, Margulies SS (2006) Stretch increases alveolar epithelial permeability to uncharged micromolecules. Am J Physiol Cell Physiol 290:C1179–C1188
Tschumperlin DJ, Oswari J, Margulies SS (2000) Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am J Respir Crit Care Med 162:357–362
Dreyfuss D, Basset G, Soler P, Saumon G (1985) Intermittent positive-pressure hyperventilation with high inflation pressures produces pulmonary microvascular injury in rats. Am Rev Respir Dis 132:880–884
Martynowicz MA, Walters BJ, Hubmayr RD (2001) Mechanisms of recruitment in oleic acid-injured lungs. J Appl Physiol 90:1744–1753
Bilek AM, Dee KC, Gaver DP 3rd (2003) Mechanisms of surface-tension-induced epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol 94:770–783
Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR (1998) Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338:347–354
Peevy KJ, Hernandez LA, Moise AA, Parker JC (1990) Barotrauma and microvascular injury in lungs of nonadult rabbits: effect of ventilation pattern. Crit Care Med 18:634–637
Ware LB, Matthay MA (2001) Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 163:1376–1383
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was supported by a grant from the French Académie Nationale de Médecine
Rights and permissions
About this article
Cite this article
de Prost, N., Roux, D., Dreyfuss, D. et al. Alveolar edema dispersion and alveolar protein permeability during high volume ventilation: effect of positive end-expiratory pressure. Intensive Care Med 33, 711–717 (2007). https://doi.org/10.1007/s00134-007-0575-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0575-5