Abstract
Objective
Neurally adjusted ventilatory assist uses the electrical activity of the diaphragm (EAdi)—a pneumatically-independent signal—to control the timing and pressure of the ventilation delivered, and should not be affected by leaks. The aim of this study was to evaluate whether NAVA can deliver assist in synchrony and proportionally to EAdi after extubation, with a leaky non-invasive interface.
Design and setting
Prospective, controlled experimental study in an animal laboratory.
Animals
Ten rabbits, anesthetized, mechanically ventilated.
Interventions
Following lung injury, the following was performed in sequential order: (1) NAVA delivered via oral endotracheal tube with PEEP; (2) same as (1) without PEEP; (3) non-invasive NAVA at unchanged NAVA level and no PEEP via a single nasal prong; (4) no assist; (5) non-invasive NAVA at progressively increasing NAVA levels.
Measurements and results
EAdi, esophageal pressure, blood gases and hemodynamics were measured during each condition. For the same NAVA level, the mean delivered pressure above PEEP increased from 3.9 ± 1.4 cmH2O (intubated) to 7.5 ± 3.8 cmH2O (non-invasive) (p < 0.05) because of increased EAdi. No changes were observed in PaO2 and PaCO2. Increasing the NAVA level fourfold during non-invasive NAVA restored EAdi and esophageal pressure swings to pre-extubation levels. Triggering (106 ± 20 ms) and cycling-off delays (40 ± 21 ms) during intubation were minimal and not worsened by the leak (95 ± 13 ms and 33 ± 9 ms, respectively).
Conclusion
NAVA can be effective in delivering non-invasive ventilation even when the interface with the patient is excessively leaky, and can unload the respiratory muscles while maintaining synchrony with the subject's demand.
Similar content being viewed by others
Introduction
The primary goals of mechanical ventilation are to unload the respiratory muscles and to provide adequate gas exchange. In spontaneously breathing patients, it is also important to achieve patient–ventilator synchrony; that is, the ventilator needs to cycle-on in unison with the onset of the patient's effort, the level of assist delivered should correspond to the patient's demand, and, most importantly, the ventilator breath should be cycled-off at the end of the patient's inspiratory effort.
It is generally believed that today's “patient-triggered” modes of ventilation are synchronous with patient effort. However, recent studies in intubated adults [1, 2] and infants [3] show that patient–ventilator dyssynchrony is common. Adverse consequences of patient–ventilator dyssynchrony have been described, including increased need for sedation or muscle paralysis [4–6], increased peak transpulmonary pressures [4] potentially causing lung injury [7], high risk for pneumothorax [8], interference with natural breathing pattern [3, 9, 10], and prolonged use of mechanical ventilation [2]. In the presence of a leak, such as with the use of uncuffed endotracheal tubes or non-invasive ventilation, modes triggered and cycled-off by flow or pressure are often asynchronous with patient effort [11] and frequently fail [12], leading to re-intubation.
Neurally adjusted ventilatory assist (NAVA) is a mode of ventilation that uses the electrical activity of the diaphragm (EAdi) to control the ventilator [13]. The EAdi represents the neural effort, both with respect to timing and amplitude [14]. Being an electrical signal, the EAdi is pneumatically independent.
With NAVA, the pressure applied at the airway opening is cycled-on when the respiratory centers initiate the breath. NAVA then delivers assist in proportion to the EAdi, until the neural activation to the diaphragm decreases and the ventilator assist is terminated [13]. In intubated rabbits with lung injury, NAVA efficiently unloads the respiratory muscles [15, 16], and offers more synchronous ventilation than conventional pressure support ventilation [16].
Since the control of assist with NAVA is theoretically not affected by leaks in the patient–ventilator interface, the aim of the present study was to evaluate whether NAVA delivered via a single nasal prong can unload the respiratory muscles during respiratory failure. A second objective was to evaluate the impact of applying non-invasive NAVA on diaphragm activity, breathing pattern, gastric distension, and cardiac performance.
This study was presented in abstract format at the American Thoracic Society Meeting in 2005 [17].
Methods
The study was approved by the St. Michael's Hospital Animal Care and Use Committee. Care and handling of the animals was in accord with the guidelines of the Canadian Council on Animal Care.
Animals and measurements
Ten anesthetized, spontaneously breathing white New Zealand rabbits with a mean body weight of 3.6 ± 0.4 kg (Charles River Laboratories, St Constant, QC, Canada) were studied (see ESM for anesthetic protocol).
Arterial blood pressure (Pd 23, Gould Inc, Cleveland, Ohio) and blood for measurement of arterial blood gases (Ciba-Corning Model 248, Bayer, Leverkusen, Germany) were obtained from an ear artery.
Oxygen saturation and heart rate were measured with pulse oximetry (NONIN 8600 V™; Nonin Medical Inc., Plymouth, MN, USA). The animals were initially orally intubated [18] with a cuffed endotracheal tube (2.5 mm OD) and ventilated by a Servo300 ventilator (Maquet Critical Care, Solna, Sweden) modified to allow the application of NAVA.
Flow and tidal volume signals were obtained from the ventilator. Airway pressure (Paw) was measured at the Y-piece of the respiratory circuit. EAdi was recorded from an array of electrodes mounted on an 8-F esophageal catheter, which also contained balloons for measurement of esophageal (Pes) and gastric (Pga) pressures. Details of the electrode configuration and catheter positioning are presented in the ESM.
Cardiac output was measured with thermodilution at the end of each experimental period using a standard cardiac output module (Datex-Ohmeda, Finland) as previously described [19].
General principles of NAVA
NAVA uses the EAdi to control the timing and magnitude of pressure delivered by the ventilator (SV300, Maquet, Solna, Sweden). The magnitude of the assist is obtained by multiplying the EAdi by a proportionality constant called the NAVA level, the units of which are cmH2O per unit of EAdi [13]. With NAVA, the assist is triggered when the EAdi exceeds a threshold increment, and is cycled-off when the EAdi falls below a set percentage (70% in this study) of peak inspiratory activity. Details about theimplementation of NAVA have been previously described [15].
Experimental protocol
Figure 1 describes the steps of the protocol. Arterial blood gas samples were drawn and cardiac output measurements made after steps 1, 3, 5, 7, and 8. FIO2 was 50% in all steps except where indicated.
-
Step 1:
(baseline period) NAVA before acute lung injury (ALI) with oral endotracheal intubation (n = 10). The rabbits were ventilated with NAVA for 20 min (NAVA level 0.5 PEEP = 2 cmH2O, FIO2 21%) and baseline recordings were obtained for the last 5 min.
-
Step 2:
Lung injury (n = 10). Lung injury was induced by instilling hydrochloric acid (2 ml/kg, pH 2.0) into the trachea via the endotracheal tube during neuromuscular paralysis. Details of the lung injury protocol have recently been published [15, 16] and are presented in the ESM.
-
Step 3:
NAVA level 1, post ALI with endotracheal intubation (n = 10). After recovery from the neuromuscular paralysis (approximately 20–30 min), theanimals were ventilated on NAVA and a PEEP titration was performed according to Allo et al. [15]; see ESM. The animals were ventilated with NAVA level 1.
-
Step 4:
Brief removal of PEEP during intubation (n = 10). PEEP was briefly removed (∼30 s) and a cardiac output measurement was repeated.
-
Step 5:
NAVA level 1, post ALI with single nasal prong (n = 10). The animals were then extubated and ventilated with NAVA level 1, via a cut Portex tube (2.5 mm OD) placed ∼2 cm into one nostril with zero PEEP for 5 min.
-
Step 6:
NAVA at lowest level post ALI with nasal prong (n = 10). The NAVA level was lowered to zero and no PEEP for 5 min.
-
Step 7:
No assist (n = 10). The animal was disconnected from the ventilator (no assist, no PEEP, no FIO2).
-
Step 8:
Progressively increasing NAVA level with nasal prong (n = 8). In this period, the NAVA level was progressively increased every 2 min.
Evaluation of tracheal pressure transmission
In five additional animals, we simultaneously measured the ventilator-delivered pressure at the nasal prong (Pvent) and in the trachea (Ptr) in order to evaluate the efficiency of pressure transmission during non-invasive NAVA at increasing NAVA levels. A tracheotomy was performed and endotracheal tubes were inserted caudally and distally into the airway with a Pneumotach (dead space 1.3 ml) placed in between to measure Ptr and tracheal flow.
Analysis
Analysis of EAdi and the other recorded respiratory variables was performed off-line. For each condition, an average of each variable was calculated for the last 1 min of the 5-min runs.
Statistical analysis was performed with Sigmastat 3.2 (Jandel Scientific, San Rafael, CA, USA). For each parameter analyzed, one-way repeated-measures analysis of variance was performed for STEPS 1, 3, 5, 6, and 7 (n = 10).
In the eight animals tested for progressively increasing gain, one-way repeated-measures analysis of variance was performed for STEPS 1, 3, 5, 6, 7, and 8. All pairwise multiple comparisons were performed with the Tukey test.
The impact of extubation itself on synchrony (n = 10) was compared for steps 3 and 5 using paired t-tests.
In the additional five animals, linear regression analysis was performed between Pvent and Ptr during non-invasive NAVA for all breaths and the regression slope and correlation coefficient were calculated.
A significant difference was defined as p < 0.05.
Results
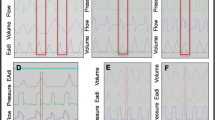
Following induction of lung injury, the mean PaO2/FIO2 ratio was 123 ± 28 and respiratory system compliance was decreased by 37 ± 12%. All animals survived the protocol. The PEEP required to minimize the tonic EAdi with the oral intubation was 8.5 ± 3.3 cmH2O. During non-invasive NAVA (at NAVA level 1), 80.9 ± 7.7% of the inspired volume was lost. Figure 2 provides examples of tracings from one lung-injured animal breathing on NAVA while intubated on the titrated PEEP level (leftmost tracing).
Examples of tracings from one representative lung-injured animal breathing on NAVA. From left to right: orally intubated with titrated PEEP, NAVA level 1; removal of PEEP; extubation (indicated by vertical line); NAVA with nasal prong at level 0; NAVA level 1; and NAVA level 4. Eadi, Diaphragm electrical activity; Paw, airway pressure; Pes, esophageal pressure
For the same NAVA level (NAVA level 1), extubation resulted in an increase in phasic (inspiratory) diaphragm activity (from 4.2 ± 1.5 to 6.4 ± 2.6 a.u.) and esophageal pressure swings (from –1.2 ± 1.0 to –3.0 ± 1.5 cmH2O) (Fig. 2, middle tracing to right of vertical line), albeit non-significant for the group (Table 1). The ventilator-delivered pressure increased from 3.9 ± 1.4 cmH2O to 7.5 ± 3.8 cmH2O (p < 0.05) (Table 1). Inspiratory time increased (p < 0.05) and respiratory rate decreased (p < 0.05) for the same NAVA level after extubation. Arterial PO2 and PCO2 were not significantly different for the same NAVA level before and after extubation. Triggering (106 ± 20 ms) and cycling-off delays (40 ± 21 ms) during intubation were minimal and not worsened by the leak (95 ± 13 ms and 33 ± 9 ms, respectively).
After extubation, when the animals were removed from the ventilator completely, arterial PO2 decreased significantly to 47 ± 9 mmHg, while phasic diaphragm electrical activity and esophageal pressure swings were approximately four times higher (p < 0.05) than observed during the baseline, pre-injury condition.
During the period of progressively increasing NAVA levels (n = 8), the phasic diaphragm activity and esophageal pressure swings were systematically reduced to the values observed during the orally intubated pre-lung injury period (Fig. 3). The mean ventilator-delivered pressure required to achieve this was 14.4 ± 6.3 cmH2O, with a 3.8 ± 1.7 time increase in the NAVA level (Fig. 3).
During intubation, immediately upon removal of PEEP, there was an instantaneous increase in the tonic EAdi, which for the group represented a 2.5-fold increase (p < 0.05) (Table 1). Following extubation, the tonic EAdi was reduced and was not different from the period with intubation and titrated PEEP (NS) or before ALI, despite the fact that no PEEP was applied. Subsequent changes in the NAVA level did not affect the tonic EAdi (Table 1, Fig. 2).The result of removing PEEP during intubation was significant increases in cardiac stroke volume, cardiac output, and mean arterial blood pressure, while mean pulmonary artery pressure decreased (p < 0.05 for all) (Table 1). All hemodynamic parameters remained unchanged following extubation, independent of the NAVA level (Table 1).
The baseline gastric pressure (data available in seven animals) during non-invasive NAVA was not influenced by increasing levels of pressure delivered through the nasal prong (6.8 ± 1.2 cmH2O while off the ventilator and 6.7 ± 1.7 cmH2O at the highest NAVA level). End-expiratory esophageal pressure was also not affected by the progressive increase in NAVA level, but was significantly lower than that measured during the oral intubation/high PEEP period (p < 0.05) (Fig. 2).
A strong correlation was found between Pvent and Ptr for all breaths in the additional five animals (mean r = 0.88 ± 0.9) (p < 0.001), regardless of the respiratory drive or the NAVA level (Fig. 4). The mean regression slope for all animals indicated that Ptr was 30 ± 7 % of Pvent. The mean Ptr at the high NAVA level was 3.5 ± 1.3 cmH2O.
Discussion
The present study is the first to demonstrate that with NAVA, subject–ventilator synchrony can be maintained even when an excessively leaky interface is used, regardless of the level of assist delivered. NAVA delivered non-invasively was able to (1) maintain synchrony and gas exchange (despite removing PEEP), (2) unload the respiratory muscles and (3) prevent gastric distension despite application of relatively high pressures. The study also demonstrates the role of removing PEEP during endotracheal intubation and non-invasive ventilation on the phasic and tonic EAdi, breathing pattern, and hemodynamics in rabbits with acute lung injury.
Upon extubation an increase in the NAVA level was required, since delivery of pressure with a single nasal prong and with an open mouth represents a severe leak. Consequently, the animal's respiratory drive increased due to the loss in pressure delivery. Despite the fact that the leak did not influence the timing of NAVA, the leak still had an impact on pressure delivery, where we observed 30% of the pressure reaching the trachea (Fig. 4) in a subgroup of animals. This efficiency of pressure delivery to the trachea was independent of the respiratory drive and the NAVA level. [Hence, the impact of the small dead space (1.3 ml) of our Pneumotach measuring system should not influence the results]. However, in order to compensate for the reduced efficiency of assist delivery in the presence of a leak, it was necessary to increase the NAVA level by approximately 4 times to achieve the same amount of unloading as occurred when the animals were intubated post ALI. It should be noted that the impact of a fourfold increase in NAVA level is not necessarily a fourfold increase in pressure delivered [16], because, during NAVA, there is no pressure or volume target; rather, the animal's respiratory control mechanisms limit pressure delivery by adjusting the respiratory drive. This means that even though the NAVA level was increased fourfold in the present study, the pressure delivered was only doubled because the animal reduced respiratory drive progressively as the NAVA level was increased. This is notably different from other conventional modes (such as pressure support), which target a fixed pressure or volume, independent of changes in respiratory drive.
In infants, a number of approaches have been used to trigger the ventilator using methods other than detection of pressure or flow at the airway opening. Barrington et al. used abdominal movement as the trigger by employing a pressure-sensitive air-filled balloon attached to a pressure transducer (Graseby capsule) taped to an infant's abdomen just below the xiphoid process [20]. The assist is triggered when the sensor detects a change in pressure in the capsule. The inspiratory time is fixed and set by the caregiver, and therefore there is no physiological signal for cycling-off the assist. A fixed inspiratory time on the ventilator may result in cycling-off that is too early or too late [3] and is probably worse in infants, given the high variability of the breathing pattern in the newborn [21]. Delivery of ventilation into neural expiration prolongs expiration in mechanically ventilated infants [3], and inspiratory time affectsrespiratory rate in the preterm [22]. Too long an inspiratory time may cause excessive delivery of pressure or volume, with the risk of inducing lung injury [7] or pneumothorax [6]. Schulze et al. [23] have described a methodusing respiratory inductance plethysmography (RIP) to control both the triggering and the cycling-off of the ventilator. In rabbits with meconium injury, they demonstrated that RIP could provide an adequate signal to the ventilator, as assessed by gas exchange and ventilation. However, the authors did caution that calibration of the RIP technology may be sensitive to body movement and chest wall distortion. To our knowledge, neither the Graseby capsule nor the RIP technology have been evaluated with respect to timing with EAdi or Pes.
The presence of patient–ventilator asynchrony in intubated infants has previously been reported [3, 24] for flow-triggered and time-cycled ventilation. In the presence of a leak around the endotracheal tube, the asynchrony with intubation can be expected to worsen with the severity of leak. In older infants, a recent short-term study demonstrated that non-invasive CPAP and BIPAP are associated with a significant and comparable decrease in respiratory effort in infants with upper airway obstruction. However, BIPAP ventilation was associated with severe patient–ventilator asynchrony [11].
The clinical impact of asynchrony during non-invasive ventilation has not yet been described. One could speculate that, in a milder form, asynchrony might cause gastric distension [25], as air is forced into the belly rather than the lung, particularly when the assist is cycled-off too late and flow continues to be delivered by the ventilator while the patient is already exhaling. In its early use, non-invasive positive-pressure ventilation could even be associated with gastric rupture [26]. The fact that gastric pressure did not increase during NAVA was likely due to the fact that the pressure was being delivered in synchrony with the respiratory muscles' contraction, drawing air into the lung when the airway is “prepared” neurally to receive inspiratory flow (glottis/upper airways dilate in synchrony with the breath). Recently, Moreau-Bussiere et al. assessed laryngeal muscle response to non-invasive pressure support and volume control in non-sedated newborn lambs and observed active glottal closure [27], suggesting “fighting of the ventilator” during conventional ventilation delivered non-invasively.
Intubation bypasses the glottis and therefore interferes with the maintenance of end-expiratory lung volume (EELV). With an endotracheal tube in place, the glottal constrictor muscles may be electrically activated in an attempt to maintain EELV [28], but their contraction around the endotracheal tube cannot increase end-expiratory resistance. When no PEEP is applied, one compensating mechanism for the loss of laryngeal braking is that the diaphragm “takes over” the role of maintaining EELV during intubation, as is evidenced by the instantaneous increase in tonic activity of the diaphragm when the PEEP was removed in the present study (Fig. 2). Allo et al. [15] and Emeriaud et al. [29] have shown that removal of PEEP results in increased tonic diaphragm activity in intubated lung-injured rabbits and mechanically ventilated infants, respectively. When PEEP is applied, the tonic EAdi is reduced or minimized ( [15, 29] (Fig. 2, Table 1). In the present study, after extubation, the tonic EAdi remained low for the group of 10 animals, suggesting that the upper airway mechanisms could participate in the maintenance of EELV and that the diaphragm could “rest” on expiration.
We used New Zealand white rabbits, with acid-induced ALI mimicking the physiological characteristics of ALI due to pneumonitis following aspiration of gastric content in adults or of meconium in newborns. The physiological characteristics of this lung injury model include severe hypoxia (as demonstrated in Table 1) reduced dynamic compliance [15, 16], surfactant dysfunction [30], increased edema [31], and, when combined with injurious mechanical ventilation, secondary organ failure [31].
The single nasal prong was the chosen interface for the present study as it is suitable for the anatomy of the rabbit model and represents a very large leak. Evidently, for the adult population, the interface used (single nasal prong) may be replaced by another non-invasive interface used more commonly in adult intensive care units. Obviously, using an interface with less of a leak would increase the efficiency of non-invasive ventilation with NAVA.
Conclusion
NAVA can be effective in delivering non-invasive ventilation even when the interface with the patient is excessively leaky, and can unload the respiratory muscles while maintaining synchrony with the subject's demand and maintaining cardiac performance. Non-invasive ventilation with NAVA may be an alternative to endotracheal intubation in hypoxemic respiratory failure.
References
Beck J, Gottfried SB, Navalesi P, Skrobik Y, Comtois N, Rossini M, Sinderby C (2001) Electrical activity of the diaphragm during pressure support ventilation in acute respiratory failure. Am J Respir Crit Care Med 164:419–424
Thille AW, Rodriguez P, Cabello B, Lellouche F, Brochard L (2006) Patient–ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med 32:1515–1522
Beck J, Tucci M, Emeriaud G, Lacroix J, Sinderby C (2004) Prolonged neural expiratory time induced by mechanical ventilation in infants. Pediatr Res 55:747–754
Stark AR, Bascom R, Frantz ID III (1979) Muscle relaxation in mechanically ventilated infants. J Pediatr 94:439–443
Henry GW, Stevens DC, Schreiner RL, Grosfeld JL, Ballantine TV (1979) Respiratory paralysis to improve oxygenation and mortality in large newborn infants with respiratory distress. J Pediatr Surg 14:761–767
Greenough A, Wood S, Morley CJ, Davis JA (1984) Pancuronium prevents pneumothoraces in ventilated premature babies who actively expire against positive pressure inflation. Lancet 1:1–3
Tremblay LN, Slutsky AS (1998) Ventilator-induced injury: from barotrauma to biotrauma. Proc Assoc Am Physicians 110:482–488
Greenough A, Morley C, Davis J (1983) Interaction of spontaneous respiration with artificial ventilation in preterm babies. J Pediatr 103:769–773
Younes M, Kun J, Webster K, Roberts D (2002) Response of ventilator-dependent patients to delayed opening of exhalation valve. Am J Respir Crit Care Med 166:21–30
Kondili E, Prinianakis G, Anastasaki M, Georgopoulos D (2001) Acute effects of ventilator settings on respiratory motor output in patients with acute lung injury. Intensive Care Med 27:1147–1157
Essouri S, Nicot F, Clement A, Garabedian EN, Roger G, Lofaso F, Fauroux B (2005) Noninvasive positive pressure ventilation in infants with upper airway obstruction: comparison of continuous and bilevel positive pressure. Intensive Care Med 31:574–580
Calderini E, Confalonieri M, Puccio PG, Francavilla N, Stella L, Gregoretti C (1999) Patient–ventilator asynchrony during noninvasive ventilation: the role of expiratory trigger. Intensive Care Med 25:662–667
Sinderby C, Navalesi P, Beck J, Skrobik Y, Comtois N, Friberg S, Gottfried SB, Lindstrom L (1999) Neural control of mechanical ventilation in respiratory failure. Nat Med 5:1433–1436
Lourenco RV, Cherniack NS, Malm JR, Fishman AP (1966) Nervous output from the respiratory center during obstructed breathing. J Appl Physiol 21:527–533
Allo JC, Beck JC, Brander L, Brunet F, Slutsky AS, Sinderby CA (2006) Influence of neurally adjusted ventilatory assist and positive end-expiratory pressure on breathing pattern in rabbits with acute lung injury. Crit Care Med 34:2997–3004
Beck J, Campoccia F, Allo JC, Brander L, Brunet F, Slutsky AS, Sinderby C (2007) Improved synchrony and respiratory unloading by neurally adjusted ventilatory assist (NAVA) in lung-injured rabbits. Pediatr Res 61:289–294
Beck J, Brander L, Allo JC, Brunet F, Slutsky AS, Reilly MC, Dunn MS, Sinderby C (2005) Non-invasive neurally adjusted ventilatory assist in rabbits with acute lung injury. Proceedings of the American Thoracic Society (PATS) 2(abstracts issue):A847
Tran HS, Puc MM, Tran JL, Del Rossi AJ, Hewitt CW (2001) A method of endoscopic endotracheal intubation in rabbits. Lab Anim 35:249–252
Franz AR, Mack C, Reichart J, Pohlandt F, Hummler HD (2001) Preserved spontaneous breathing improves cardiac output during partial liquid ventilation. Am J Respir Crit Care Med 164:36–42
Barrington KJ, Bull D, Finer NN (2001) Randomized trial of nasal synchronized intermittent mandatory ventilation compared with continuous positive airway pressure after extubation of very low birth weight infants. Pediatrics 107:638–641
Al Hathlol K, Idiong N, Hussain A, Kwiatkowski K, Alvaro RE, Weintraub Z, Cates DB, Rigatto H (2000) A study of breathing pattern and ventilation in newborn infants and adult subjects. Acta Paediatr 89:1420–1425
Upton CJ, Milner AD, Stokes GM (1990) The effect of changes in inspiratory time on neonatal triggered ventilation. Eur J Pediatr 149:648–650
Schulze A, Suguihara C, Gerhardt T, Schaller P, Claure N, Everett R, Bancalari E (1998) Effects of respiratory mechanical unloading on thoracoabdominal motion in meconium-injured piglets and rabbits. Pediatr Res 43:191–197
Hird MF, Greenough A (1991) Spontaneous respiratory effort during mechanical ventilation in infants with and without acute respiratory distress. Early Hum Dev 25:69–73
Abdel-Hady H, Mohareb S, Khashaba M, Abu-Alkhair M, Greisen G (1998) Randomized controlled trial of discontinuation of nasal-CPAP in stable preterm infants breathing room air. Acta Paediatr 87:82–87
Garland JS, Nelson DB, Rice T, Neu J (1985) Increased risk of gastrointestinal perforations in neonates mechanically ventilated with either face mask or nasal prongs. Pediatrics 76:406–410
Moreau-Bussiere F, Samson N, St Hilaire M, Reix P, Lafond JR, Nsegbe E, Praud JP (2007) Laryngeal response to nasal ventilation in nonsedated newborn lambs. J Appl Physiol 102:2149–2157
Hutchison AA, Wozniak JA (2000) Endotracheal measurement of thyroarytenoid activity in newborn lambs. Biol Neonate 78:139–144
Emeriaud G, Beck J, Tucci M, Lacroix J, Sinderby C (2006) Diaphragm electrical activity during expiration in mechanically ventilated infants. Pediatr Res 59:705–710
Brackenbury AM, Puligandla PS, McCaig LA, Nikore V, Yao LJ, Veldhuizen RA, Lewis JF (2001) Evaluation of exogenous surfactant in HCL-induced lung injury. Am J Respir Crit Care Med 163:1135–1142
Imai Y, Parodo J, Kajikawa O, de Perrot M, Fischer S, Edwards V, Cutz E, Liu M, Keshavjee S, Martin TR, Marshall JC, Ranieri VM, Slutsky AS (2003) Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA 289:2104–2112
Acknowledgements
The authors are indebted to Norman Comtois for his assistance during the experiments.
Conflict of interest. Disclosure for Jennifer Beck and Christer Sinderby
JB and CS have been reimbursed by Maquet Critical Care (Solna, Sweden) for attending several conferences; JB and CS have participated as speakers in scientific meetings or courses organized and financed by Maquet Critical Care; JB and CS through Neurovent Research, serve as consultants to Maquet Critical Care. The following disclosure was agreed upon by University of Toronto, Sunnybrook Health Science Centre, St-Michael's Hospital and the REBs of Sunnybrook and St-Michael's to resolve conflicts of interest:
“Dr Beck and Dr Sinderby have made inventions related to neural control of mechanical ventilation that are patented. The license for these patents belongs to Maquet Critical Care. Future commercial uses of this technology may provide financial benefit to Dr Beck and Dr Sinderby through royalties. Dr Beck and Dr Sinderby each own 50% of Neurovent Research Inc (NVR). NVR is a research and development company that builds the equipment and catheters for research studies. NVR has a consulting agreement with Maquet Critical Care. Dr Beck and Dr Sinderby are married.”
Disclosure for Arthur Slutsky
Consultancies: “Dr Slutsky is a paid consultant to Maquet.”
Institutional conflict of interest: “St-Michael's Hospital will get a small royalty if Maquet includes SMH-patented discoveries in the ventilator.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial support J.B. and C.S. were supported by the NIH # 1 R21 HD45047-01. L.B. held postdoctoral fellowships from the Swiss Foundation for Fellowships in Medicine and Biology (SSMBS) provided by Novartis AG and from the Division of Respirology at the University of Toronto provided by Merck-Frosst. Supported in part by the Canadian Institutes for Health Research (CIHR) and the Canada Foundation for Innovation (CFI).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Beck, J., Brander, L., Slutsky, A.S. et al. Non-invasive neurally adjusted ventilatory assist in rabbits with acute lung injury. Intensive Care Med 34, 316–323 (2008). https://doi.org/10.1007/s00134-007-0882-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0882-x